2. 福建中医药大学药学院, 福建 福州 350122
2. School of Pharmacy, Fujian University of Traditional Chinese Medicine, Fuzhou 350122, China
泽泻Alismatis Rhizoma为泽泻科植物泽泻Alisma orientalis (Sam.) Juzep. 的干燥块茎。中医理论认为其甘、淡,寒,归肾、膀胱经,利水渗湿,泄热,化浊降脂。从20世纪60年代起至今,国内外学者从泽泻中分离得到的化学成分有近百种,以原萜烷型四环三萜类成分为主要成分[1]。现代药理学研究也表明,泽泻具有较好的降糖作用[2, 3, 4, 5],但其药效物质基础尚未完全阐明。为了进一步研究泽泻降糖作用的物质基础,本实验对泽泻水提物和醇提物进行体内降糖活性考察,在明确其降糖作用的基础上对提取物进行化学成分研究。对高脂饲喂致小鼠胰岛素抵抗模型,泽泻水、醇提物均可明显改善糖耐量。对泽泻醇提物进行化学成分研究,共分离得到16个化合物,分别鉴定为谷甾醇(sitosterol,1)、棕榈酸(palmitic acid,2)、十七烷酸(heptadecanoic acid,3)、二十烷酸(eicosanoic acid,4)、11-去氧泽泻醇B(11-deoxy-alisol B,5)、23-乙酰泽泻醇B(23-acetate alisol B,6)、23-乙酰泽泻醇C(23-acetate alisol C,7)、泽泻醇B(alisol B,8)、24-乙酰泽泻醇A(24-acetate alisol A,9)、泽泻醇G(alisol G,10)、24-乙酰泽泻醇F(24-acetate alisol F,11)、泽泻醇L(alisol L,12)、泽泻醇C(alisol C,13)、泽泻醇F(alisol F,14)、泽泻醇A(alisol A,15)、16-羰基-24-乙酰泽泻醇A(16-oxo-24-acetate alisol A,16)。其中化合物3、4为首次从泽泻中分离得到,泽泻三萜类成分可能是其降糖药效物质基础之一。
1 仪器和材料Bruker AEANCE III核磁共振仪(德国布鲁克公司);Waters SQD2 MS(美国Waters公司);Waters Auto Purification自动纯化系统制备液相(美国Waters公司);中低压制备色谱仪(苏州利穗公司);色谱柱(福州裕华实验器材有限公司);薄层色谱硅胶、柱色谱硅胶(青岛海洋化工有限公司);Sephadex LH-20凝胶、ODS-C18反相填料50 μm(北京绿百草有限公司);血糖测定仪购自江苏鱼跃医疗设备股份有限公司;FLUO STAR Omega多功能荧光酶标仪(德国BMG LABTECH公司);HERACELL 150i型CO2恒温培养箱(美国Thermo公司);2-NBDG(美国Invitron公司);罗格列酮(美国Sigma公司);胎牛血清、胰酶、DMEM低糖(美国HycLone公司),其余所用试剂均为分析纯。
泽泻干燥块茎购自福建金山医药有限公司,经福建中医药大学药用植物实验室范世明高级实验师鉴定为泽泻科植物泽泻Alisma orientalis (Sam.) Juzep. 的干燥块茎。
8周龄SPF级雄性C57BL/6J小鼠,许可证号SCXK(京)2009-0004,购自北京华阜康生物科技股份有限公司;普通饲料(5%脂肪)、高脂饲料(45%脂肪)及高脂添加泽泻水提物、醇提物饲料均由广东省医学实验动物中心加工制作。人肝癌HepG2细胞(中国科学院上海细胞库)。
2 方法与结果 2.1 泽泻提取物制备泽泻粗粉2 kg用8倍量的80%乙醇溶液加热回流提取3次,时间均为2 h,抽滤得提取液,减压回收溶剂得泽泻醇提物200 g。另取泽泻粗粉1 kg用8倍量的蒸馏水,加热回流提取3次,时间均为2 h,抽滤得提取液,减压回收溶剂得泽泻水提物100 g。
2.2 高脂饮食诱导小鼠胰岛素抵抗模型及泽泻干预作用雄性C57BL/6J小鼠40只,体质量(15.0±1.2)g,饲养于福建医科大学SPF级实验动物中心。适应性喂养1周后,将小鼠随机分为对照组(ND组)、高脂饮食组(HFD组)、泽泻水提物干预组(ZXS组)、泽泻醇提物干预组(ZXC组),每组10只,ND组给予普通饲料,HFD组给予高脂饲料,ZXS组和ZXC组前8周先给予高脂饲料造模,在第9周进行糖耐量实验验证造模成功后,ZXS组给予泽泻水提物混合高脂饲料(相当于每千克高脂饲料含泽泻生药量100 g的水提物,按进食量换算给药量为生药量12 g/kg),ZXC组则给予泽泻醇提物混合高脂饲料(相当于每千克高脂饲料含泽泻生药量100 g的醇提物,按进食量换算给药量为生药量12 g/kg),每周记录体质量,在小鼠喂养至第11周时,进行糖耐量实验:实验前小鼠禁食,然后ip葡萄糖溶液(1.5 g/kg),并于0、15、30、60、120 min尾静脉取血,并用血糖仪测定血糖浓度值;所得血糖值-时间的曲线下面积AUC由DAS 2.0软件分析得出。从AUC统计分析结果表明泽泻醇提物和水提物均能有效改善糖耐量曲线,结果见图 1、2,表明泽泻醇、水提物均有降糖作用。
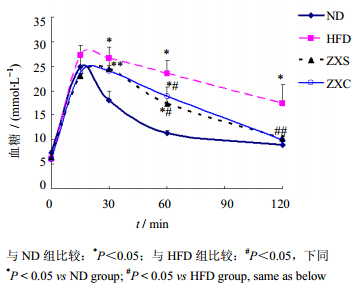 | 图 1 泽泻提取物对高脂饲喂小鼠糖耐量的影响(x±s,n=10)Fig. 1 Effect of A. orientalis extracts on HFD-induced abnormality mice in glucose tolerance(x±s,n=10) |
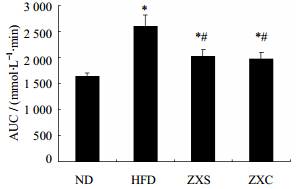 | 图 2 各组葡萄糖耐量血糖值-时间曲线下面积(x±s,n=10)Fig. 2 Area under curve of glucose tolerance test(x±s,n=10) |
将泽泻醇提物100 g,用硅胶拌样,以不同比例石油醚-醋酸乙酯(50∶1→0∶1)梯度洗脱,得到5个洗脱部分Fr. 1~5(50∶1、10∶1、4∶1、1∶1、0∶1),其中Fr. 1采用硅胶柱色谱法分离,以石油醚-醋酸乙酯(50∶1→15∶1)的不同比例的洗脱液经反复硅胶柱色谱分别得到化合物1(85 mg)、2(15 mg)、3(20 mg)、4(24 mg)。Fr. 2采用硅胶柱色谱分离,以石油醚-醋酸乙酯(10∶1→1∶1)不同比例的洗脱液经TLC跟踪检识,馏份经Sephadex LH-20凝胶柱色谱法分离得到化合物5(20 mg)。Fr. 3采用硅胶柱色谱分离,以石油醚-醋酸乙酯(5∶1→1∶1)洗脱,流分经中压-ODS色谱制备得化合物6(585 mg)、化合物7(383 mg)。Fr. 4采用中压-ODS色谱制备分离和Waters自动纯化系统制备液相制备,得到化合物8(255 mg)、9(120 mg)、10(12 mg)、11(8 mg)。Fr. 5采用中压-ODS色谱制备分离和Waters自动纯化系统制备液相制备,得到化合物12(20 mg)、13(22 mg)、14(10 mg)、15(55 mg)、16(11 mg)。
2.4 结构鉴定化合物1:无色针状结晶(醋酸乙酯),硫酸乙醇加热显红色,与β-谷甾醇对照品共薄层,斑点Rf值相同。ESI-MS m/z: 437 [M+Na]+,分子式C29H50O,相对分子质量为414。1H-NMR (400 MHz,CDCl3) δ: 3.45 (1H,ddd,J = 6.4,10.8,16.0 Hz,H-3),5.37 (1H,d,J = 5.2 Hz,H-4);13C-NMR (100 MHz,CDCl3) δ: 37.2 (C-1),31.5 (C-2),71.8 (C-3),42.3 (C-4),140.7 (C-5),121.7 (C-6),31.6 (C-7),31.9 (C-8),50.1 (C-9),36.5 (C-10),21.1 (C-11),28.2 (C-12),42.2 (C-13),56.7 (C-14),24.3 (C-15),39.6 (C-16),55.9 (C-17),12.2 (C-18),19.4 (C-19),36.1 (C-20),18.7 (C-21),33.9 (C-22),26.0 (C-23),45.8 (C-24),29.1 (C-25),19.0 (C-26),19.8 (C-27),23.0 (C-28),11.8 (C-29)。以上数据与文献报道基本一致[6],故鉴定化合物1为β-谷甾醇。
化合物2:白色粒状结晶(石油醚-醋酸乙酯),硫酸乙醇显紫红色,ESI-MS m/z: 279 [M+Na]+,分子式C16H32O2,相对分子质量256。1H-NMR (400 MHz,CDCl3) δ: 0.88 (3H,t,J = 6.8 Hz,H-16),1.28 (2H×12,brs,H-4~15),1.63 (2H,m,H-3),2.34 (2H,t,J = 7.6 Hz,H-2);13C-NMR (100 MHz,CDCl3) δ: 180.1 (C-1),34.1 (C-2),31.9 (C-3),29.7 (C-4),29.6~29.0 (C-5~13),24.7 (C-14),22.7 (C-15),14.1 (C-16)。以上数据与文献报道基本一致[7],故鉴定化合物2为棕榈酸。
化合物3:白色粉末,5%的硫酸乙醇显紫红色,ESI-MS m/z: 293 [M+Na]+,分子式C17H34O2,相对分子质量270。1H-NMR (400 MHz,CDCl3) δ: 0.88 (3H,t,J = 6.8 Hz,H-17),1.28 (2H×13,brs,H-4~16),1.63 (2H,m,H-3),2.34 (2H,t,J = 7.2 Hz,H-2);13C-NMR (100 MHz,CDCl3) δ: 180.1 (C-1),34.1 (C-2),31.9 (C-3),29.72 (C-4),29.7~29.1 (C-5~14),24.7 (C-15),22.7 (C-16),14.1 (C-17)。以上数据与文献报道基本一致[8],故鉴定化合物3为十七烷酸。
化合物4:白色粉末状结晶(石油醚-醋酸乙酯),硫酸乙醇显紫红色。ESI-MS m/z: 335 [M+Na]+,分子式C20H40O2,相对分子质量312。1H-NMR (400 MHz,CDCl3) δ: 0.88 (3H,t,J = 6.4 Hz,H-20),1.28 (2H×16,brs,H-4~19),1.63 (2H,m,H-3),2.34 (2H,t,J = 7.2 Hz,H-2);13C-NMR (100 MHz,CDCl3) δ: 180.1 (C-1),34.1 (C-2),31.9 (C-3),29.7 (C-4),29.7~29.1 (C-5~17),24.7 (C-18),22.7 (C-19),14.1 (C-20)。以上数据与文献报道基本一致[9],故鉴定化合物4为二十烷酸。
化合物5:白色粉末,硫酸乙醇显紫色,Liebermann-Burchard反应显阳性。ESI-MS m/z: 479 [M+Na]+,分子式C30H48O3,相对分子质量456。1H-NMR (400 MHz,CDCl3) δ: 0.81,1.00,1.06,1.07,1.20,1.23,1.31 (各3H,s,30,19,28,29,18,26,27-CH3),1.03 (3H,d,J = 7.2 Hz,H-21),2.47 (1H,dd,J = 5.2,14.0 Hz,H-12),2.69 (1H,d,J = 8.4 Hz,H-24),3.23 (1H,m,H-23);13C-NMR (100 MHz,CDCl3) δ: 31.9 (C-1),33.8 (C-2),220.2 (C-3),47.0 (C-4),48.2 (C-5),20.0 (C-6),34.0 (C-7),40.6 (C-8),43.9 (C-9),36.2 (C-10),22.6 (C-11),22.9 (C-12),140.4 (C-13),57.3 (C-14),31.1 (C-15),28.8 (C-16),133.7 (C-17),23.5 (C-18),22.8 (C-19),27.7 (C-20),20.2 (C-21),38.9 (C-22),69.3 (C-23),67.8 (C-24),59.2 (C-25),19.1 (C-26),24.7 (C-27),29.1 (C-28),19.7 (C-29),23.6 (C-30)。以上数据与文献报道基本一致[10],故鉴定化合物5为11-去氧泽泻醇B。
化合物6:无色棱晶(甲醇),硫酸乙醇液加热显紫红色斑点,Liebermann-Burchard反应显阳性。ESI-MS m/z: 537 [M+Na]+,分子式C32H50O5,相对分子质量514。1H-NMR (400 MHz,CDCl3) δ: 0.96,1.04,1.05,1.06,1.14,1.31,1.33 (各3H,s,30,19,28,29,18,26,27-CH3),1.02 (3H,d,J = 7.2 Hz,H-21),1.72 (1H,d,J = 10.8 Hz,H-9),2.06 (3H,s,OAc),2.55 (1H,dd,J = 5.6,13.2 Hz,H-12),2.73 (1H,d,J = 8.4 Hz,H-24),3.80 (1H,m,H-11),4.61 (1H,m,H-23);13C-NMR (100 MHz,CDCl3) δ: 30.9 (C-1),33.7 (C-2),220.0 (C-3),46.9 (C-4),48.4 (C-5),20.0 (C-6),34.1 (C-7),40.7 (C-8),50.0 (C-9),36.9 (C-10),70.2 (C-11),34.5 (C-12),138.0 (C-13),57.0 (C-14),30.6 (C-15),29.1 (C-16),134.2 (C-17),23.1 (C-18),25.6 (C-19),27.8 (C-20),20.0 (C-21),36.7 (C-22),71.4 (C-23),65.0 (C-24),58.4 (C-25),19.4 (C-26),24.6 (C-27),29.5 (C-28),20.1 (C-29),23.8 (C-30),169.9 (C-31),21.1 (C-32)。以上数据与文献报道基本一致[10],故鉴定化合物6为23-乙酰泽泻醇B。
化合物7:无色柱晶(甲醇),硫酸乙醇液加热显无色斑点,置于365 nm紫外灯下显示蓝色斑点,Liebermann- Burchard反应显阳性。ESI-MS m/z: 551 [M+Na]+,分子式C32H48O6,相对分子质量528。1H-NMR (400 MHz,CDCl3) δ: 0.89,1.07,1.08,1.10,1.23,1.29,1.32 (各3H,s,30,29,28,19,26,27,18-CH3),1.19 (3H,d,J = 6.8 Hz,H-21),1.76 (1H,ddd,J = 4.0,10.8,14.8 Hz,H-22),1.88 (1H,d,J = 11.2 Hz,H-9),2.06 (3H,s,OAc),2.62 (1H,m,H-20),2.72 (1H,d,J = 8.4 Hz,H-24),2.89 (1H,dd,J = 5.6,14.0 Hz,H-12),4.00 (1H,m,H-11),4.52 (1H,m,H-23);13C-NMR (100 MHz,CDCl3) δ: 30.8 (C-1),33.5 (C-2),220.0 (C-3),46.9 (C-4),48.4 (C-5),19.9 (C-6),34.8 (C-7),40.1 (C-8),48.8 (C-9),36.9 (C-10),69.8 (C-11),35.6 (C-12),176.8 (C-13),49.7 (C-14),45.7 (C-15),207.9 (C-16),138.5 (C-17),23.0 (C-18),25.5 (C-19),26.6 (C-20),20.0 (C-21),35.6 (C-22),71.8 (C-23),64.9 (C-24),58.6 (C-25),19.7 (C-26),24.6 (C-27),29.5 (C-28),19.2 (C-29),23.1 (C-30),170.0 (C-31),21.1 (C-32)。以上数据与文献报道基本一致[10],故鉴定化合物7为23-乙酰泽泻醇C。
化合物8:白色无定形粉末,硫酸乙醇液显黄色,Liebermann-Burchard反应显阳性。ESI-MS m/z: 495 [M+Na]+,分子式C30H48O4,相对分子质量472。1H-NMR (400 MHz,CDCl3) δ: 0.99,1.05,1.06,1.07,1.12,1.24,1.31 (各3H,s,30,19,28,29,18,26,27-CH3),1.02 (3H,d,J = 6.8 Hz,H-21),1.55 (1H,ddd,J = 3.6,10.4,14.0 Hz,H-22α),1.73 (1H,d,J = 10.8 Hz,H-9),2.69 (1H,d,J = 8.4 Hz,H-24),2.79 (1H,dd,J = 5.6,13.2 Hz,H-12),2.90 (1H,m,H-22β),3.21 (1H,m,H-23),3.87 (1H,ddd,J = 5.6,10.4,10.8 Hz,H-11);13C-NMR (100 MHz CDCl3) δ: 31.0 (C-1),33.7 (C-2),220.2 (C-3),46.9 (C-4),48.5 (C-5),20.0 (C-6),34.3 (C-7),40.6 (C-8),49.7 (C-9),36.9 (C-10),69.9 (C-11),34.4 (C-12),138.0 (C-13),57.0 (C-14),30.6 (C-15),29.1 (C-16),134.9 (C-17),23.3 (C-18),25.5 (C-19),27.7 (C-20),20.1 (C-21),38.8 (C-22),69.1 (C-23),67.8 (C-24),59.2 (C-25),19.1 (C-26),24.9 (C-27),29.5 (C-28),20.0 (C-29),23.9 (C-30)。以上数据与文献报道基本一致[10],故鉴定化合物8为泽泻醇B。
化合物9:无色针晶(乙腈),硫酸乙醇液显红色斑点,Liebermann-Burchard反应显阳性。ESI-MS m/z: 555 [M+Na]+,分子式C32H52O6,相对分子质量532。1H-NMR (400 MHz,CDCl3) δ: 0.99,1.00,1.06,1.07,1.14,1.16,1.30 (各3H,s,18,19,29,30,28,26,27-CH3),0.99 (3H,d,J = 6.8 Hz,H-21),1.75 (1H,d,J = 10.8 Hz,H-9),2.19 (3H,s,OAc),2.81 (2H,m,H-12,20),3.85 (2H,overlapped,H-11,23),4.60 (1H,brd,J = 1.2 Hz,H-24);13C-NMR (100 MHz CDCl3) δ: 31.0 (C-1),33.7 (C-2),220.1 (C-3),46.9 (C-4),48.5 (C-5),20.0 (C-6),34.3 (C-7),40.5 (C-8),49.7 (C-9),36.9 (C-10),70.0 (C-11),34.5 (C-12),137.9 (C-13),57.0 (C-14),30.5 (C-15),29.1 (C-16),135.2 (C-17),23.2 (C-18),25.5 (C-19),27.9 (C-20),19.9 (C-21),39.7 (C-22),69.0 (C-23),78.7 (C-24),73.8 (C-25),27.4 (C-26),26.5 (C-27),29.5 (C-28),20.0 (C-29),24.1 (C-30),170.8 (C-31),20.7 (C-32)。以上数据与文献报道基本一致[10],故鉴定化合物9为24-乙酰泽泻醇A。
化合物10:白色粉末,ESI-MS m/z: 495 [M+Na]+,分子式C30H48O4,相对分子质量472。1H-NMR (400 MHz CDCl3) δ: 0.99,1.01,1.06,1.07,1.14 (各3H,s,30,19,29,28,18,26-CH3),1.68 (3H,s,H-27),1.00 (3H,d,J = 7.2 Hz,H-21),1.74 (1H,d,J = 10.8,H-9),2.82 (1H,dd,J = 5.2,13.2 Hz,H-12),3.35 (1H,ddd,J = 3.2,6.4,10.0 Hz,H-23),3.79 (1H,d,J = 6.4 Hz,H-24),3.88 (1H,m,H-11),4.95 (1H,brs,H-26a),4.99 (1H,brs,H-26b);13C-NMR (100 MHz,CDCl3) δ: 30.6 (C-1),33.7 (C-2),220.1 (C-3),46.9 (C-4),48.5 (C-5),20.3 (C-6),34.6 (C-7),40.6 (C-8),49.7 (C-9),36.9 (C-10),70.1 (C-11),34.2 (C-12),137.5 (C-13),57.0 (C-14),31.0 (C-15),29.1 (C-16),135.5 (C-17),23.3 (C-18),25.6 (C-19),28.3 (C-20),20.0 (C-21),38.3 (C-22),70.5 (C-23),79.5 (C-24),144.5 (C-25),114.0 (C-26),17.9 (C-27),29.5 (C-28),20.0 (C-29),24.0 (C-30)。以上数据与文献报道基本一致[10],故鉴定化合物10为泽泻醇G。
化合物11:白色粉末,硫酸乙醇液显蓝色斑点,ESI-MS m/z: 553 [M+Na]+,分子式C32H50O6,相对分子质量530。1H-NMR (400 MHz,CDCl3) δ: 0.85,1.06,1.06,1.07,1.12,1.24,1.37 (各3H,s,30,19,29,28,18,26,27-CH3),1.16 (3H,d,J = 7.2 Hz,H-21),1.75 (1H,d,J = 10.8 Hz,H-9),2.19 (3H,s,OAc),2.70 (1H,m,H-12),2.81 (1H,m,H-20),3.79 (1H,m,H-11),4.27 (1H,brd,J = 12.0 Hz,H-23),4.46 (1H,dd,J = 5.2,8.0 Hz,H-16),4.74 (1H,brd,J = 2.4 Hz,H-24);13C-NMR (100 MHz,CDCl3) δ: 30.7 (C-1),33.5 (C-2),219.8 (C-3),46.9 (C-4),48.1 (C-5),19.8 (C-6),33.8 (C-7),40.5 (C-8),49.6 (C-9),36.9 (C-10),70.5 (C-11),34.3 (C-12),137.3 (C-13),55.3 (C-14),39.1 (C-15),80.9 (C-16),132.5 (C-17),24.2 (C-18),25.5 (C-19),26.6 (C-20),18.2 (C-21),34.0 (C-22),72.5 (C-23),77.3 (C-24),72.8 (C-25),26.6 (C-26),27.8 (C-27),29.6 (C-28),20.0 (C-29),23.6 (C-30),171.2 (C-31),20.8 (C-32)。以上数据与文献报道基本一致[11],故鉴定化合物11为24-乙酰泽泻醇F。
化合物12:白色粉末,ESI-MS m/z: 491 [M+Na]+,分子式C30H44O4,相对分子质量468。1H-NMR (400 MHz,CDCl3) δ: 0.97,0.97,1.08,1.12,1.12,1.19,1.32 (各3H,s,19,30,29,28,18,26,27-CH3),1.25 (3H,d,J = 7.2 Hz,H-21),2.41 (1H,d,J = 10.8 Hz,H-9),2.71 (1H,d,J = 8.0 Hz,H-24),3.02 (1H,m,H-22),3.14 (1H,m,H-23),6.18 (1H,dd,J = 2.0,10.0 Hz,H-12),6.72 (1H,dd,J = 3.2,10.0 Hz,H-11);13C- NMR (100 MHz,CDCl3) δ: 32.2 (C-1),33.3 (C-2),219.0 (C-3),47.2 (C-4),46.1 (C-5),19.2 (C-6),31.2 (C-7),39.1 (C-8),48.1 (C-9),36.0 (C-10),138.5 (C-11),122.1 (C-12),171.5 (C-13),47.7 (C-14),44.4 (C-15),208.0 (C-16),138.2 (C-17),24.1 (C-18),24.9 (C-19),25.8 (C-20),19.4 (C-21),38.4 (C-22),69.4 (C-23),67.5 (C-24),59.0 (C-25),19.3 (C-26),24.7 (C-27),29.2 (C-28),19.2 (C-29),21.9 (C-30)。以上数据与文献报道基本一致[12],故鉴定化合物12为泽泻醇L。
化合物13:白色粉末,硫酸乙醇液加热显无色斑点,置于365 nm紫外灯下显示蓝色斑点。ESI-MS m/z: 509 [M+Na]+,分子式C30H46O5,相对分子质量486。1H-NMR (400 MHz,CDCl3) δ: 0.91,1.07,1.08,1.11,1.13,1.16,1.29 (各3H,s,30,29,28,19,26,27,18-CH3),1.23 (3H,d,J = 7.2 Hz,H-21),1.89 (1H,d,J = 10.8 Hz,H-9),2.69 (1H,d,J = 8.0 Hz,H-24),2.92 (1H,m,H-20),3.16 (1H,dd,J = 5.6,14.0 Hz,H-12),3.06 (1H,m,H-23),4.05 (1H,m,H-11);13C-NMR (100 MHz,CDCl3)δ: 30.8 (C-1),33.5 (C-2),219.3 (C-3),46.9 (C-4),48.4 (C-5),19.9 (C-6),34.9 (C-7),39.9 (C-8),48.6 (C-9),36.9 (C-10),69.4 (C-11),35.7 (C-12),176.5 (C-13),49.7 (C-14),45.5 (C-15),208.1 (C-16),139.3 (C-17),23.2 (C-18),25.4 (C-19),26.2 (C-20),20.0 (C-21),37.7 (C-22),69.7 (C-23),67.6 (C-24),59.3 (C-25),19.4 (C-26),24.8 (C-27),29.5 (C-28),19.1 (C-29),23.5 (C-30)。以上数据与文献报道基本一致[10],故鉴定化合物13为泽泻醇C。
化合物14:白色粉末,硫酸乙醇液显蓝色斑点。ESI-MS m/z: 511 [M+Na]+,分子式C30H48O5,相对分子质量488。1H-NMR (400 MHz,CDCl3) δ: 0.90,1.08,1.09,1.10,1.26,1.27,1.32 (各3H,s,30,19,29,28,18,26,27-CH3),1.19 (3H,d,J = 7.2 Hz,H-21),1.76 (1H,d,J = 10.8 Hz,H-9),2.66 (1H,dd,J = 5.6,12.4 Hz,H-12),2.86 (1H,m,H-20),3.07 (1H,brd,J = 8.0 Hz,H-24),3.82 (1H,ddd,J = 5.6,10.8,10.8 Hz,H-11),4.05 (1H,brd,J = 11.6 Hz,H-23),4.48 (1H,dd,J = 4.8,8.8 Hz,H-16);13C-NMR (100 MHz,CDCl3) δ: 30.7 (C-1),33.9 (C-2),219.8 (C-3),46.9 (C-4),48.2 (C-5),19.9 (C-6),33.6 (C-7),40.5 (C-8),49.6 (C-9),36.9 (C-10),70.5 (C-11),34.9 (C-12),136.9 (C-13),55.3 (C-14),39.4 (C-15),80.1 (C-16),133.1 (C-17),24.4 (C-18),25.4 (C-19),26.6 (C-20),18.3 (C-21),33.9 (C-22),72.6 (C-23),77.2 (C-24),73.3 (C-25),26.5 (C-26),27.0 (C-27),29.6 (C-28),20.0 (C-29),23.6 (C-30)。以上数据与文献报道基本一致[13],故鉴定化合物14为泽泻醇F。
化合物15:白色粉末,硫酸乙醇液显红色斑点,Liebermann-Burchard反应显阳性。ESI-MS m/z: 513 [M+Na]+,分子式C30H46O5,相对分子质量490。1H-NMR (400 MHz,CDCl3) δ: 1.00,1.06,1.06,1.07,1.14,1.22,1.27 (各3H,s,18,19,29,30,28,26,27-CH3),1.01 (3H,d,J = 6.8 Hz,H-21),1.66 (1H,m,H-22),1.75 (1H,d,J = 10.8 Hz,H-9),2.79 (1H,dd,J = 5.6,13.2 Hz,H-12),3.01 (1H,brs,H-24),3.79 (1H,dd,J = 3.2,9.6 Hz,H-23),3.88 (1H,m,H-11);13C-NMR (100 MHz,CDCl3) δ: 31.0 (C-1),33.7 (C-2),220.2 (C-3),46.9 (C-4),48.5 (C-5),20.0 (C-6),34.3 (C-7),40.5 (C-8),49.7 (C-9),36.9 (C-10),70.0 (C-11),34.5 (C-12),137.5 (C-13),57.0 (C-14),30.5 (C-15),29.2 (C-16),135.6 (C-17),23.1 (C-18),25.6 (C-19),28.3 (C-20),20.1 (C-21),40.0 (C-22),69.4 (C-23),77.5 (C-24),74.2 (C-25),27.3 (C-26),26.0 (C-27),29.5 (C-28),20.0 (C-29),24.1 (C-30)。以上数据与文献报道基本一致[10],故鉴定化合物15为泽泻醇A。
化合物16:白色粉末,ESI-MS m/z: 569 [M+Na]+,分子式C32H50O7,相对分子质量546。1H-NMR (400 MHz,CDCl3) δ: 0.89,1.07,1.08,1.12,1.13,1.24,1.33 (各3H,s,18,19,29,30,28,26,27-CH3),1.16 (3H,d,J = 6.4 Hz,H-21),1.94 (1H,d,J = 9.6 Hz,H-9),2.20 (3H,s,OAc),3.20 (1H,dd,J = 6.0,14.0 Hz,H-12),3.79 (1H,dd,J = 2.4,9.2 Hz,H-23),4.05 (1H,m,H-11),4.60 (1H,brd,J = 1.2 Hz,H-24);13C-NMR (100 MHz,CDCl3) δ: 31.9 (C-1),33.5 (C-2),219.5 (C-3),46.9 (C-4),48.4 (C-5),20.8 (C-6),34.9 (C-7),40.1 (C-8),48.7 (C-9),36.9 (C-10),69.8 (C-11),39.4 (C-12),177.4 (C-13),50.1 (C-14),45.6 (C-15),209.4 (C-16),140.0 (C-17),22.9 (C-18),25.3 (C-19),25.6 (C-20),20.0 (C-21),36.1 (C-22),72.6 (C-23),78.4 (C-24),69.5 (C-25),27.1 (C-26),26.8 (C-27),29.5 (C-28),19.2 (C-29),23.4 (C-30),170.9 (C-31),21.3 (C-32)。以上数据与文献报道基本一致[10],故鉴定化合物16为16-羰基-24乙酰泽泻醇A。
3 泽泻三萜体外降糖活性实验泽泻三萜5~16,分别采用MTT法测定对HepG2细胞的安全给药浓度,其中11-去氧泽泻醇B(5)、23-乙酰泽泻醇C(7)、24-乙酰泽泻醇F(11)、泽泻醇L(12)、泽泻醇C(13)、泽泻醇F(14)、16-羰基-24-乙酰泽泻醇A(16)及阳性药罗格列酮(Ros)最终选择给药浓度为10和20 μmol/L,而23-乙酰泽泻醇B(6)、泽泻醇B(8)、24-乙酰泽泻醇A(9)、泽泻醇G(10)、泽泻醇A(15)最终选择5和10 μmol/L,在上述浓度下各化合物对HepG2细胞活力无影响。三萜化合物体外降糖活性评价采用基于HepG2细胞的2-NBDG法[14, 15, 16, 17],2-NBDG [2-(N-7-硝基-2,1,3-苯并恶二唑-4-氨基)-2-脱氧葡萄糖] 是带荧光标记的葡萄糖类似物。将HepG2细胞以每孔1×104个细胞密度接种至96孔荧光板,用DMEM低糖培养基配制上述浓度的泽泻单体化合物处理24 h后,更换培养液,使用DMEM低糖培养液(含100 μmol/L 2-NBDG)培养90 min,弃上清,PBS清洗3次后加入70 μL含1% Triton X-100 PBS液,避光裂解细胞10 min后,加30 μL DMSO振荡摇匀后立即于荧光酶标仪λex 485 nm和λem 520 nm处检测(同时检测背景荧光强度,即无细胞处理组)。通过比较给药后细胞荧光强度变化来反馈给药组对细胞萄葡糖摄取活性的影响,葡萄糖摄取活性=(药物组荧光强度-背景荧光强度) / (空白组荧光强度-背景荧光强度)。结果见图 3。
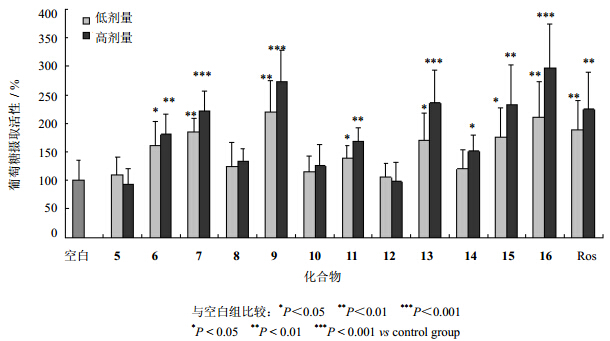 | 图 3 泽泻中三萜类化合物促进HepG2细胞葡萄糖摄取活性评价(x±s,n=6)Fig. 3 Effect of triterpenes from A. orientalis on glucose uptake in HepG2 cells(x±s,n=6) |
实验结果表明,与阳性药罗格列酮相似,泽泻三萜类成分,如16-羰基-24乙酰泽泻醇A(16)、24-乙酰泽泻醇A(9)、泽泻醇C(13)、泽泻醇A(15)等9个三萜化合物对细胞葡萄糖摄取均具有明显的促进作用,而11-去氧泽泻醇B(5)、泽泻醇B(8)和泽泻醇G(10)对细胞葡萄糖摄取作用不明显。
4 讨论泽泻在古医籍上的记载很多,被《神农本草经》列为上品,临床上用于调脂、化浊、利尿等,对于泽泻的降糖作用的研究也有报道,杨新波等[18, 19, 20]报道了泽泻提取物降糖作用,其选择的模型主要是链脲佐菌素(STZ)或者四氧嘧啶造成胰岛细胞的破坏后的高血糖症,针对的是I型糖尿病模型,泽泻提取物表现良好的降糖作用。另外,对于泽泻三萜的降糖作用的研究目前报道较少,Lin等[3]考察了3种泽泻三萜成分对法尼醇受体(FRX)的作用,结果表明23-乙酰泽泻醇M和23-乙酰泽泻醇A能通过FRX途径起降糖作用;Li等[4]研究表明泽泻三萜类化合物:泽泻醇F、泽泻醇B、24-乙酰泽泻醇A具有α-葡萄糖苷酶抑制作用,其并采用体外细胞葡萄糖消耗模型评价泽泻三萜提取物,表明其具有显著促进葡萄糖吸收的活性,但未阐明其活性单体成分。本实验首先通过模拟正常高脂饮食的高脂饲料诱导建立C57BL/6J小鼠胰岛素抵抗模型[21],采用温和的自然喂养泽泻提取物干预模型小鼠,结果表明泽泻水提和醇提物对于胰岛素抵抗的II型糖尿病均有很好的作用,该药效作用目前还未见报道。在此基础上,针对泽泻醇提物进行化学成分研究,从中分离鉴定16种成分,所得化合物主要为泽泻三萜类成分,采用2-NBDG葡萄糖摄取法评价所得三萜单体促进葡萄糖摄取活性,表明多种泽泻三萜均具有促进葡萄糖摄取活性,因此,初步认为三萜类成分可能是泽泻降糖作用的药效物质基础之一。此外,本研究还发现泽泻水提物也具有非常好的降糖作用,也将对其药效物质基础进行进一步研究。
| [1] | Liu X, Li S L, Zhou Y, et al. Characterization of protostane triterpenoids in Alisma orientalis by ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry [J]. Rapid Commun Mass Spectrom, 2010, 24(11): 1514-1522. |
| [2] | Tadakazu M, Yoshio I, Takeo H, et al. Biological-active triterpenes of Alismatis Rhizoma. I. Isolation of the alisols [J]. Chem Pharm Bull, 1970, 18(7): 1347-1353. |
| [3] | Lin H R. Triterpenes from Alisma orientalis act as farnesoid X receptor agonists [J]. Bioorg Med Chem Lett, 2012, 22(14): 4787-4792 |
| [4] | Li Q, Qu H B. Study on the hypoglycemic activities and metabolism of alcohol extract of Alismatis Rhizoma [J]. Fitoterapia, 2012, 83(6): 1046-1053. |
| [5] | Hisashi M, Tadashi K, Iwao T, et al. Effects of sesquiterpenes and triterpenes from the rhizome of Alisma orientale on nitric oxide production in lipopolysaccharide- activated macrophages: absolute stereostructures of Alismaketones-B 23-acetate and C 23-acetate [J]. Bioorg Med Chem Lett, 1999, 9(21): 3081-3086. |
| [6] | 杨 杰, 王丽莉, 周鑫堂, 等. 黄花草木犀化学成分研究 [J]. 中草药, 2014, 45(6): 622-625. |
| [7] | 于宗贵, 刘建宇, 宫益霞, 等. 朝鲜白头翁非皂苷类化学成分研究 [J]. 中草药, 2013, 44(23): 3264-3269. |
| [8] | 李火云, 焦 珂, 张 鹏, 等. 拟缺香茶菜化学成分研究 [J]. 中草药, 2014, 45(2): 154-160. |
| [9] | 陈晓东, 田 伟, 邓仁华, 等. 那藤化学成分研究 [J]. 中草药, 2013, 44(6): 671-673. |
| [10] | Nakajima Y, Satoh Y, Katsumata M, et al. Terpenoids of Alisma orientate Rhizome and the crude drug Alismatis rhizome [J]. Phytochemistry, 1994, 36(1): 119-127. |
| [11] | 周雪峰, 尹仁杰, 阮汉利, 等. 泽泻抑制尿草酸钙结石形成活性成分的2D NMR分析 [J]. 波谱学杂志, 2005, 22(2): 196-200. |
| [12] | Masayuki Y, Norimichi T, Toshiyuki M, et al. Studies on Alismatis Rhizoma. Ⅲ. Stereostructures of new protostane-type triterpenes, Alisols H, I, J-23-acetate, K-23-acetate, L-23-acetate, M-23-acetate, and N-23-acetate, from the dried rhizome of Alisma orientale [J]. Chem Pharm Bull, 1999, 47(4): 524-528. |
| [13] | Hu X Y, Guo Y Q, Gao W Y, et al. Two new triterpenes from the rhizomes of Alisma orientalis [J]. J Asian Nat Prod Res, 2008, 10(5): 481-484. |
| [14] | Wang L, Xu M L, Rasmussen S K, et al. Vomifoliol 9-O-a-arabinofuranosyl (1→6)-β-D-glucopyranoside from the leaves of Diospyros Kakistimulates the glucose uptake in HepG2 and 3T3-L1 cells [J]. Carbohydr Res, 2011, 346(10): 1212-1216. |
| [15] | Ha D T, Trung T N, Hien T T, et al. Selected compounds derived from Moutan Cortex stimulated glucose uptake and glycogen synthesisvia AMPK activation in human HepG2 cells [J]. J Ethnopharmacol, 2010, 131(2): 417-424. |
| [16] | Vidyashankar S, Sharath K L M, Barooah V, et al. Liv.52 up-regulates cellular antioxidants and increase glucose uptake to circumvent oleic acid induced hepatic steatosis in HepG2 cells [J]. Phytomedicine, 19(13): 1156-1165. |
| [17] | Jung D W, Ha H H, Zheng X X, et al. Novel use of fluorescent glucose analogues to identify a new class of triazine-based insulin mimetics possessing useful secondary effects [J]. Mol Bio Syst, 2011, 7(2): 346-358. |
| [18] | 杨新波, 黄正明, 曹文斌, 等. 泽泻提取物对链脲佐菌素高血糖小鼠的治疗和保护作用 [J]. 解放军药学学报, 2002, 18(6): 336-350. |
| [19] | 杨新波, 黄正明, 曹文斌, 等. 泽泻提取物对正常及四氧嘧啶小鼠糖尿病模型的影响 [J]. 中国实验方剂学杂志, 2002, 8(3): 24-26. |
| [20] | 杨新波, 黄正明, 陈红艳, 等. 泽泻不同溶剂提取物对糖尿病小鼠血糖及血液生化指标的影响 [J]. 解放军药学学报, 2006, 22(6): 419-421. |
| [21] | Yu Z, Shao W, Chiang Y, et al. Oltipraz upregulates the nuclear respiratory factor 2 alpha subunit (NRF2) antioxidant system and prevents insulin resistance and obesity induced by a high-fat diet in C57BL/6J mice [J]. Diabetologia, 2011, 54(4): 922-934. |
 2014, Vol. 49
2014, Vol. 49


