2. 中国医学科学院北京协和医学院药用植物研究所, 北京 100193
2. Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100193, China
西松烷型二萜是一类母核骨架具有十四元环的天然产物,其结构特点是在母核骨架上连有一个异丙基和3个甲基,结构变化主要是双键的位移、碳环化以及母核骨架氧化程度不同[1-3]。该类天然产物多存在于低等海洋生物中,陆生植物中相对少见[4-5]。因该类天然产物结构新颖、多样并具有广泛的生物活性,现已成为天然产物化学与药物化学研究的热点方向[6-7]。大量研究表明,西松烷型二萜具有抗炎[8-10]、抗菌[11-17]、抗HIV[18-19]和抗肿瘤[20-29]等活性。其中,在抗肿瘤方面表现出的显著活性已越来越受到人们的关注。
光叶巴豆Croton laevigatus Vahl.系大戟科巴豆属植物,别名抱龙(傣名),分布于云南、广东、海南岛等地[30],根、叶入药,为傣族常用药,味辛,性温,入肝、胃经,具有通经活血、散癖消肿、退热止痛之功效,既可内服也可外用,常用于治疗治跌打损伤、骨折、疟疾、胃痛等症[31-32]。本课题组前期研究发现光叶巴豆叶醇提取物具有一定的抗肿瘤活性,并且在化学成分研究中分离得到一系列西松烷型二萜化合物(图 1)。对得到的该类天然产物进行了抗肿瘤活性研究发现,其中,新巴豆瑞士松酸对HeLa肿瘤细胞株表现出较强的抑制作用(IC50=45.4 μmol/L)[33-34]。此外,新巴豆瑞士松酸在该植物中的量很高(0.70%,100 g生药),这为基于该化合物进行结构修饰提供了很好的物质基础。基于上述工作基础,本实验以天然产物新巴豆瑞士松酸为研究对象,运用药物设计的拼合原理,通过对其20位的羧酸基团进行酰胺化,将具有抗肿瘤活性的三氮唑基团引入到该天然产物中,合成了11个未见文献报道的新的取代三氮唑类酰胺衍生物。同时,通过体外抗肿瘤活性模型,首次对这些新巴豆瑞士松酸衍生物进行体外抗肿瘤活性评价,以期发现具有深入研究价值的新型抗肿瘤化合物,为中药新药的研发奠定基础。

|
图 1 从光叶巴豆叶中分离得到的西松烷型二萜化合物 Fig.1 Cembrane diterpenoids isolated from leaves of C. laevigatus |
1 仪器与试剂
新巴豆瑞士松酸(本实验室分离得到,质量分数≥95%),1-羟基苯并三唑(HOBT)、碳化二亚胺(EDCI)、对甲基溴苄、4-氯氯苄均购于北京偶合科技有限公司,2-氟溴苄购于Adams公司,4-甲氧基溴苄、4-氰基溴化苄均购于百灵威科技有限公司,叠氮化钠购于Amresco公司,对溴溴苄、对甲苯磺酰叠氮、噻吩-2-甲酸亚铜均购于安耐吉化学,其余试剂均为分析纯,购于北京市通广精细化工有限公司。
薄层色谱板GF254(烟台化工厂);快速柱色谱用硅胶(200~300目,青岛海洋化工厂)。Bruker AV Ⅲ 600核磁共振仪和Bruker AV400核磁共振仪(Bruker-Biospin);Waters SYNAPT G2 HDMS高分辨质谱仪(Waters公司)。
2 目标化合物的合成以新巴豆瑞士松酸为起始原料,在HOBT/EDCI缩合剂的作用下与炔丙胺经缩合反应生成中间体1。运用点击化学反应,在噻吩-2-甲酸亚铜的催化下与对甲苯磺酰叠氮反应,得到含有1, 2, 3-三氮唑的酰胺衍生物2a。以取代的溴苄化合物3b~3j为原料,在丙酮-水(4:1)的条件下,与叠氮化钠反应,生成取代的叠氮化合物4b~4j,再与中间体1反应生成目标化合物2b~2j。化合物2k的合成是以化合物5为原料,同样与叠氮化钠反应,得到叠氮化合物6,最后与中间体1反应生成目标化合物2k。合成路线见图 2。
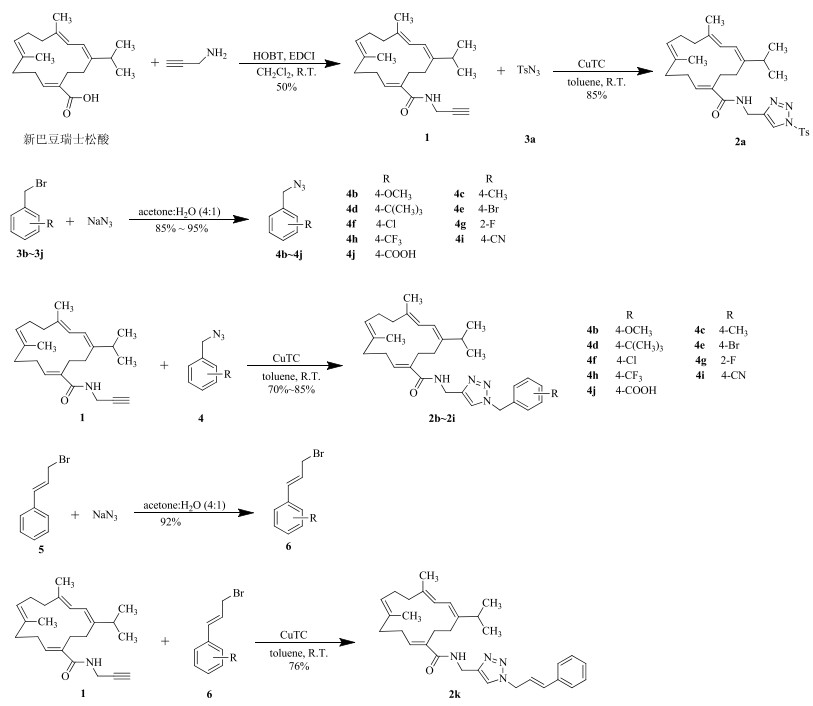
|
图 2 新巴豆瑞士松酸酰胺衍生物的合成路线 Fig.2 Synthetic routes of amide derivatives of neocrotocembraneic acid |
2.1 N-[(1-对甲基苯磺酰基)-1H-1, 2, 3-三氮唑-4-亚甲基]-新巴豆瑞士松酰胺(2a)的合成 2.1.1 N-炔丙基新巴豆瑞士松酰胺(1)的合成
将新巴豆瑞士松酸(150 mg,0.5 mmol)溶解于10 mL无水二氯甲烷中,加入HOBT(81 mg,0.6 mmol)和EDCI(115 mg,0.6 mmol),室温反应2 h,加入炔丙胺(55 μL,0.6 mmol),室温搅拌反应1 h,TLC检测反应完全,依次加入1 mol/L盐酸、饱和碳酸氢钠溶液和饱和食盐水进行洗涤,分出有机层,无水硫酸钠干燥,减压浓缩,硅胶柱色谱纯化,洗脱剂:石油醚(60~90 ℃)-醋酸乙酯10:1,得到目标产物,无色油状物,收率为65%。
2.1.2 2a的合成将炔丙基新巴豆瑞士松酰胺1(100 mg,0.3 mmol)溶于5 mL无水甲苯中,依次加入对甲苯磺酰叠氮(153 μL,0.75 mmol),噻吩-2-甲酸亚铜,室温搅拌反应1 h,TLC检测反应完全,减压蒸干溶剂,硅胶柱色谱纯化,洗脱剂为石油醚-醋酸乙酯(1:1),得到目标化合物2a,无色油状物,收率85%。HR-MS m/z537.290 0 [M+H]+(计算值537.289 9,C30H41N4O3S)。1H-NMR (600 MHz, CDCl3) δ: 8.08 (1H, s, H-23), 7.97 (2H, d, J=8.5 Hz, H-25, 29), 7.37 (2H, d, J=8.3 Hz, H-26, 28), 5.99 (1H, t, J=5.7 Hz, H-N), 5.92 (1H, s, H-2), 5.92 (1H, s, H-3), 5.78 (1H, t, J=7.6 Hz, H-11), 5.04 (1H, t, J=6.6 Hz, H-7), 4.38 (2H, d, J=5.7 Hz, H-21), 2.48 (2H, m, H-10), 2.42 (3H, s, H-30), 2.34~2.30 (1H, m, H-15), 2.30~2.27 (2H, m, H-13), 2.26~2.23 (2H, m, H-14), 2.22~2.19 (2H, m, H-6), 2.17~2.14 (2H, m, H-9), 2.14~2.12 (2H, m, H-5), 1.72 (3H, s, H-18), 1.58 (3H, s, H-19), 1.00 (3H, s, H-16), 0.98 (3H, s, H-17);13C-NMR (150 MHz, CDCl3) δ: 171.6 (C-20), 147.3 (C-1), 145.8 (C-11), 144.8 (C-27), 138.0 (C-12), 136.1 (C-4), 135.7 (C-27), 133.5 (C-24), 133.0 (C-26, 28), 130.4 (C-25, 29), 128.7 (C-22), 127.9 (C-7), 122.3 (C-23), 119.6 (C-3), 118.8 (C-2), 38.8 (C-9), 36.9 (C-21), 35.0 (C-5), 33.6 (C-15), 29.5 (C-14), 27.2 (C-13), 25.9 (C-10), 24.9 (C-6), 22.1 (C-16, 17), 21.8 (C-30), 18.2 (C-18), 16.7 (C-19)。
2.2 N-[(取代苄基)-1H-1, 2, 3-三氮唑-4-亚甲基]-新巴豆瑞士松酰胺(2b~2i)的合成 2.2.1 取代苄基叠氮4b~4i的合成(通法)将取代的溴苄(1.0 mmol)溶解于8 mL丙酮中,再加入2 mL蒸馏水,混匀,加入叠氮化钠(97 mg,1.5 mmol),室温搅拌反应1 h,TLC检测反应完全,加入30 mL水,二氯甲烷(30 mL×3)萃取,有机相用无水硫酸钠干燥,减压浓缩,硅胶柱色谱纯化,洗脱剂为石油醚-醋酸乙酯(100:1),得到目标产物4b~4i,收率为90%~95%。
2.2.2 N-[1-(4-甲氧基苄基)-1H-1, 2, 3-三氮唑-4-亚甲基]-新巴豆瑞士松酰胺(2b)的合成将化合物1(80 mg,0.24 mmol)和对甲氧基苄基叠氮4b(76 mg,0.36 mmol)溶解于5 mL无水甲苯中,加入噻吩-2-甲酸亚铜(9.1 mg,0.048 mmol),室温搅拌反应1~2 h,TLC检测反应完全,减压蒸干溶剂,硅胶柱色谱纯化,洗脱剂为石油醚-醋酸乙酯(1:1),得到目标产物,无色油状物,收率为82%。HR-MS m/z: 503.338 9 [M+H]+(计算值503.338 6,C31H43N4O2)。1H-NMR (600 MHz, CDCl3) δ: 7.44 (1H, s, H-23), 7.22 (2H, d, J=8.6 Hz, H-26, 30), 6.88 (2H, d, J=8.6 Hz, H-27, 29), 6.15 (1H, t, J=5.7 Hz, H-N), 5.92 (1H, s, H-2), 5.92 (1H, s, H-3), 5.81 (1H, t, J=7.4 Hz, H-11), 5.40 (2H, s, H-24), 5.06 (1H, t, J=6.4 Hz, H-7), 4.38 (2H, d, J=5.7 Hz, H-21), 3.80 (3H, s, H-31), 2.46 (2H, m, H-10), 2.34~2.30 (1H, m, H-15), 2.30~2.27 (2H, m, H-13), 2.27~2.24 (2H, m, H-14), 2.22~2.18 (2H, m, H-6), 2.17~2.15 (2H, m, H-9), 2.15~2.13 (2H, m, H-5), 1.73 (3H, s, H-18), 1.61 (3H, s, H-19), 1.00 (3H, s, H-16), 0.99 (3H, s, H-17);13C-NMR (150 MHz, CDCl3) δ: 171.5 (C-20), 160.1 (C-28), 146.0 (C-1), 145.4 (C-11), 138.2 (C-12), 136.1 (C-4), 135.5 (C-8), 129.9 (C-26, 30), 129.7 (C-22), 128.0 (C-7), 126.5 (C-25), 122.3 (C-23), 119.7 (C-3), 119.0 (C-2), 114.6 (C-27, 29), 55.5 (C-31), 53.9 (C-24), 39.0 (C-9), 37.1 (C-21), 35.3 (C-5), 33.7 (C-15), 29.6 (C-14), 27.6 (C-13), 26.2 (C-10), 25.0 (C-6), 22.3 (C-16, 17), 18.3 (C-18), 17.0 (C-19)。
2.2.3 N-[1-(4-甲基苄基)-1H-1, 2, 3-三氮唑-4-亚甲基]-新巴豆瑞士松酰胺(2c)的合成合成方法同“2.2.2”项2b,得无色油状物,收率70%。HR-MS m/z: 487.343 8[M+H]+(计算值487.343 7,C31H43N4O)。1H-NMR (600 MHz, CDCl3) δ: 7.43 (1H, s, H-23), 7.14 (2H, s, H-26, 30), 7.14 (2H, s, H-27, 29), 6.15 (1H, t, J=5.6 Hz, H-N), 5.91 (1H, s, H-2), 5.91 (1H, s, H-3), 5.81 (1H, t, J=7.4 Hz, H-11), 5.41 (2H, s, H-24), 5.05 (1H, t, J=6.6 Hz, H-7), 4.36 (2H, d, J=5.6 Hz, H-21), 2.45 (2H, m, H-10), 2.32 (3H, s, H-31), 2.31~2.28 (1H, m, H-15), 2.28~2.25 (2H, m, H-13), 2.25~2.22 (2H, m, H-14), 2.20~2.17 (2H, m, H-6), 2.15~2.13 (2H, m, H-9), 2.13~2.11 (2H, m, H-5), 1.71 (3H, s, H-18), 1.59 (3H, s, H-19), 0.98 (3H, s, H-16), 0.97 (3H, s, H-17);13C-NMR (150 MHz, CDCl3) δ: 171.3 (C-20), 145.8 (C-1), 145.3 (C-11), 138.7 (C-28), 138.1 (C-12), 136.0 (C-4), 135.2 (C-8), 133.6 (C-25), 131.4 (C-22), 129.7 (C-27, 29), 128.2 (C-26, 30), 127.8 (C-7), 122.2 (C-23), 119.6 (C-3), 118.8 (C-2), 54.0 (C-24), 38.3 (C-9), 37.0 (C-21), 35.2 (C-5), 33.6 (C-15), 29.4 (C-14), 27.5 (C-13), 26.0 (C-10), 24.9 (C-6), 22.1 (C-16, 17), 21.1 (C-31), 18.1 (C-18), 16.9 (C-19)。
2.2.4 N-[1-(4-叔丁基苄基)-1H-1, 2, 3-三氮唑-4-亚甲基]-新巴豆瑞士松酰胺(2d)的合成合成方法同“2.2.2”项2b,得无色油状物,收率82%。HR-MS m/z: 529.390 8 [M+H]+(计算值529.390 6,C34H49N4O)。1H-NMR (600 MHz, CDCl3) δ: 7.44 (1H, s, H-23), 7.37 (2H, d, J=8.2 Hz, H-27, 29), 7.20 (2H, d, J=8.4 Hz, H-26, 30), 6.07 (1H, t, J=5.6 Hz, H-N), 5.92 (1H, s, H-2), 5.92 (1H, s, H-3), 5.80 (1H, t, J=7.4 Hz, H-11), 5.43 (2H, s, H-24), 5.05 (1H, t, J=6.4 Hz, H-7), 4.37 (2H, d, J=5.7 Hz, H-21), 2.46 (2H, m, H-10), 2.34~2.30 (1H, m, H-15), 2.30~2.27 (2H, m, H-13), 2.26~2.23 (2H, m, H-14), 2.22~2.19 (2H, m, H-6), 2.17~2.14 (2H, m, H-9), 2.14~2.13 (2H, m, H-5), 1.72 (3H, s, H-18), 1.61 (3H, s, H-19), 1.30 (3×3H, s, H-32, 33, 34), 0.99 (3H, s, H-16), 0.98 (3H, s, H-17);13C-NMR (150 MHz, CDCl3) δ: 171.4 (C-20), 151.9 (C-28), 145.8 (C-1), 145.2 (C-11), 138.2 (C-12), 136.0 (C-4), 135.2 (C-8), 133.6 (C-25), 131.4 (C-22), 128.0 (C-26, 30), 127.9 (C-7), 126.0 (C-27, 29), 122.2 (C-23), 119.7 (C-3), 118.8 (C-2), 53.9 (C-24), 38.9 (C-9), 37.0 (C-21), 35.3 (C-5), 34.4 (C-15), 33.6 (C-31), 31.2 (C-32, 33, 34), 29.5 (C-14), 27.4 (C-13), 26.0 (C-10), 24.9 (C-6), 22.2 (C-16, 17), 18.2 (C-18), 16.9 (C-19)。
2.2.5 N-[1-(4-溴苄基)-1H-1, 2, 3-三氮唑-4-亚甲基]-新巴豆瑞士松酰胺(2e)的合成合成方法同“2.2.2”项2b,得浅黄色油状物,收率86%。HR-MS m/z: 551.238 2 [M+H]+(计算值551.238 5,C30H40BrN4O)。1H-NMR (600 MHz, CDCl3) δ: 7.49 (2H, d, J=8.4 Hz, H-27, 29), 7.47 (1H, s, H-23), 7.14 (2H, d, J=8.4 Hz, H-26, 30), 6.05 (1H, t, J=5.8 Hz, H-N), 5.93 (1H, s, H-2), 5.93 (1H, s, H-3), 5.78 (1H, t, J=7.4 Hz, H-11), 5.42 (2H, s, H-24), 5.05 (1H, t, J=6.6 Hz, H-7), 4.37 (2H, d, J=5.7 Hz, H-21), 2.47 (2H, m, H-10), 2.33~2.29 (1H, m, H-15), 2.29~2.27 (2H, m, H-13), 2.27~2.24 (2H, m, H-14), 2.22~2.18 (2H, m, H-6), 2.17~2.15 (2H, m, H-9), 2.15~2.14 (2H, m, H-5), 1.73 (3H, s, H-18), 1.62 (3H, s, H-19), 1.00 (3H, s, H-16), 0.99 (3H, s, H-17);13C-NMR (150 MHz, CDCl3)δ: 171.5 (C-20), 145.8 (C-1), 145.6 (C-11), 138.1 (C-12), 136.0 (C-4), 135.3 (C-8), 133.6 (C-25), 133.5 (C-27, 29), 132.3 (C-26, 30), 129.7 (C-22), 127.9 (C-7), 123.0 (C-23), 122.4 (C-3), 119.6 (C-28), 118.8 (C-2), 53.5 (C-24), 38.8 (C-9), 37.0 (C-21), 35.3 (C-5), 33.6 (C-15), 29.5 (C-14), 27.3 (C-13), 25.9 (C-10), 24.9 (C-6), 22.1 (C-16, 17), 18.2 (C-18), 16.8 (C-19)。
2.2.6 N-[1-(4-氯苄基)-1H-1, 2, 3-三氮唑-4-亚甲基]-新巴豆瑞士松酰胺(2f)的合成合成方法同“2.2.2”项2b,得无色油状物,收率82%。HR-MS m/z: 507.289 0 [M+H]+(计算值507.289 1,C30H40ClN4O)。1H-NMR (600 MHz, CDCl3) δ: 7.47 (1H, s, H-23), 7.33 (2H, d, J=8.4 Hz, H-27, 29), 7.20 (2H, d, J=8.5 Hz, H-26, 30), 6.04 (1H, t, J=5.8 Hz, N-H), 5.93 (1H, s, H-2), 5.93 (1H, s, H-3), 5.79 (1H, t, J=7.4 Hz, H-11), 5.44 (2H, s, H-24), 5.05 (1H, t, J=6.7 Hz, H-7), 4.37 (2H, d, J=5.7 Hz, H-21), 2.46 (2H, m, H-10), 2.33~2.30 (1H, m, H-15), 2.30~2.27 (2H, m, H-13), 2.27~2.24 (2H, m, H-14), 2.22~2.19 (2H, m, H-6), 2.17~2.15 (2H, m, H-9), 2.15~2.13 (2H, m, H-5), 1.73 (3H, s, H-18), 1.62 (3H, s, H-19), 1.00 (3H, s, H-16), 0.99 (3H, s, H-17);13C-NMR (150 MHz, CDCl3)δ: 171.5 (C-20), 145.8 (C-1), 145.6 (C-11), 138.2 (C-12), 136.0 (C-4), 135.3 (C-8), 134.9 (C-25), 133.6 (C-28), 133.0 (C-22), 129.5 (C-27, 29), 129.3 (C-26, 30), 127.9 (C-7), 122.4 (C-23), 119.7 (C-3), 118.9 (C-2), 53.4 (C-24), 38.9 (C-9), 37.0 (C-21), 35.3 (C-5), 33.6 (C-15), 29.5 (C-14), 27.4 (C-13), 26.0 (C-10), 24.9 (C-6), 22.2 (C-16, 17), 18.2 (C-18), 16.9 (C-19)。
2.2.7 N-[1-(2-氟苄基)-1H-1, 2, 3-三氮唑-4-亚甲基]-新巴豆瑞士松酰胺(2g)的合成合成方法同“2.2.2”项2b,得无色油状物,收率78%。HR-MS m/z: 491.318 2[M+H]+(计算值491.318 6,C30H40FN4O)。1H-NMR (600 MHz, CDCl3) δ: 7.52 (1H, s, H-23), 7.33 (1H, m, H-28), 7.25 (1H, td, J=7.6, 1.8 Hz, H-27), 7.12 (1H, td, J=7.6, 0.9 Hz, H-30), 7.10~7.07 (1H, m, H-29), 6.04 (1H, t, J=5.7 Hz, H-N), 5.91 (1H, s, H-2), 5.91 (1H, s, H-3), 5.79 (1H, t, J=7.5 Hz, H-11), 5.52, (2H, s, H-24), 5.04 (1H, t, J=6.7 Hz, H-7), 4.37 (2H, d, J=5.6 Hz, H-21), 2.45 (2H, m, H-10), 2.33~2.29 (1H, m, H-15), 2.28~2.25 (2H, m, H-13), 2.25~2.22 (2H, m, H-14), 2.21~2.18 (2H, m, H-6), 2.16~2.13 (2H, m, H-9), 2.13~2.11 (2H, m, H-5), 1.71 (3H, s, H-18), 1.58 (3H, s, H-19), 0.99 (3H, s, H-16), 0.98 (3H, s, H-17);13C-NMR (150 MHz, CDCl3) δ: 171.4 (C-20), 160.5 (d, J=248.2 Hz, C-26), 145.8 (C-1), 145.3 (C-11), 138.1 (C-12), 136.0 (C-4), 135.2 (C-8), 133.6 (C-22), 130.9 (d, J=8.0 Hz, C-30), 130.6 (d, J=3.1 Hz, C-28), 127.9 (C-7), 124.8 (d, J=3.8 Hz, C-29), 122.4 (C-23), 121.7 (d, J=14.6 Hz, C-25), 119.6 (C-3), 118.8 (C-2), 115.9 (d, J=21.0 Hz, C-27), 47.7 (C-24), 38.8 (C-9), 36.9 (C-21), 35.2 (C-5), 33.6 (C-15), 29.5 (C-14), 27.4 (C-13), 26.5 (C-10), 24.9 (C-6), 22.1 (C-16, 17), 18.1 (C-18), 16.8 (C-19)。
2.2.8 N-[1-(4-三氟甲基苄基)-1H-1, 2, 3-三氮唑-4-亚甲基]-新巴豆瑞士松酰胺(2h)的合成合成方法同“2.2.2”项2b,得无色油状物,收率72%。HR-MS m/z: 541.315 0 [M+H]+(计算值541.315 4,C31H40F3N4O)。1H-NMR (600 MHz, CDCl3) δ: 7.60 (2H, d, J=8.1 Hz, H-27, 29), 7.54 (1H, s, H-23), 7.36 (2H, d, J=8.0 Hz, H-26, 30), 6.21 (1H, t, J=5.8 Hz, H-N), 5.92 (1H, s, H-2), 5.92 (1H, s, H-3), 5.81 (1H, t, J=7.5 Hz, H-11), 5.52 (2H, s, H-24), 5.06 (1H, t, J=6.6 Hz, H-7), 4.38 (2H, d, J=5.8 Hz, H-21), 2.45 (2H, m, H-10), 2.32~2.28 (1H, m, H-15), 2.28~2.26 (2H, m, H-13), 2.26~2.24 (2H, m, H-14), 2.20~2.17 (2H, m, H-6), 2.16~2.14 (2H, m, H-9), 2.14~2.12 (2H, m, H-5), 1.71 (3H, s, H-18), 1.62 (3H, s, H-19), 0.98 (3H, s, H-16), 0.97 (3H, s, H-17);13C-NMR (150 MHz, CDCl3) δ: 171.4 (C-20), 145.8 (C-1), 145.7 (C-11), 138.4 (C-25), 138.0 (C-12), 135.9 (C-4), 135.4 (C-8), 135.6 (C-22), 131.0 (q, J=32.3 Hz, C-28), 128.3 (C-26, 30), 127.8 (C-7), 126.0 (q, J=3.9 Hz, C-27, 29), 125.2 (q, J=270.3 Hz, C-31), 122.7 (C-23), 119.6 (C-3), 118.8 (C-2), 53.5 (C-24), 38.8 (C-9), 37.0 (C-21), 35.1 (C-5), 33.6 (C-15), 29.4 (C-14), 27.4 (C-13), 26.0 (C-10), 24.8 (C-6), 22.1 (C-16, 17), 18.2 (C-18), 16.8 (C-19)。
2.2.9 N-[1-(4-氰基苄基)-1H-1, 2, 3-三氮唑-4-亚甲基]-新巴豆瑞士松酰胺(2i)的合成合成方法同“2.2.2”项2b,得无色油状物,收率78%。HR-MS m/z: 498.323 2 [M+H]+(计算值498.323 3,C31H40N5O)。1H-NMR (600 MHz, CDCl3) δ: 7.62 (2H, d, J=8.3 Hz, H-27, 29), 7.56 (1H, s, H-23), 7.32 (2H, d, J=8.3 Hz, H-26, 30), 6.18 (1H, t, J=5.8 Hz, H-N), 5.91 (1H, s, H-2), 5.91 (1H, s, H-3), 5.79 (1H, t, J=7.5 Hz, H-11), 5.52 (2H, s, H-24), 5.04 (1H, t, J=6.5 Hz, H-7), 4.37 (2H, d, J=5.7 Hz, H-21), 2.44 (2H, m, H-10), 2.31~2.27 (1H, m, H-15), 2.27~2.24 (2H, m, H-13), 2.24~2.22 (2H, m, H-14), 2.19~2.16 (2H, m, H-6), 2.15~2.13 (2H, m, H-9), 2.13~2.11 (2H, m, H-5), 1.70 (3H, s, H-18), 1.62 (3H, s, H-19), 0.98 (3H, s, H-16), 0.97 (3H, s, H-17);13C-NMR (150 MHz, CDCl3) δ: 171.5 (C-20), 145.8 (C-1), 145.7 (C-11), 139.7 (C-25), 138.0 (C-12), 135.9 (C-4), 135.4 (C-8), 133.6 (C-22), 132.8 (C-27, 29), 128.5 (C-26, 30), 127.8 (C-7), 122.9 (C-23), 119.6 (C-3), 118.8 (C-2), 118.1 (C-31), 112.7 (C-28), 53.3 (C-24), 38.8 (C-9), 37.0 (C-21), 35.2 (C-5), 33.6 (C-15), 29.4 (C-14), 27.4 (C-13), 26.0 (C-10), 24.8 (C-6), 22.1 (C-16, 17), 18.2 (C-18), 16.8 (C-19)。
2.2.10 N-[1-(4-甲酸苄基)-1H-1, 2, 3-三氮唑-4-亚甲基]-新巴豆瑞士松酰胺(2j)的合成合成方法同“2.2.2”项2b,得无色油状物,收率76%。HR-MS m/z: 517.318 1 [M+H]+(计算值517.317 9,C31H41N4O3)。1H-NMR (600 MHz, CDCl3) δ: 8.04 (2H, d, J=8.3 Hz, H-27, 29), 7.72 (1H, s, H-23), 7.33 (2H, d, J=8.3 Hz, H-26, 30), 6.68 (1H, t, J=5.9 Hz, H-N), 5.91 (1H, s, H-2), 5.91 (1H, s, H-3), 5.89 (1H, t, J=7.5 Hz, H-11), 5.55 (2H, s, H-24), 5.08 (1H, t, J=6.5 Hz, H-7), 4.45 (2H, d, J=5.9 Hz, H-21), 2.44 (2H, m, H-10), 2.34~2.29 (1H, m, H-15), 2.29~2.25 (2H, m, H-13), 2.25~2.22 (2H, m, H-14), 2.20~2.17 (2H, m, H-6), 2.16~2.14 (2H, m, H-9), 2.14~2.12 (2H, m, H-5), 1.71 (3H, s, H-18), 1.63 (3H, s, H-19), 0.98 (3H, s, H-16), 0.97 (3H, s, H-17);13C-NMR (150 MHz, CDCl3) δ: 171.6 (C-20), 169.1 (C-31), 145.7 (C-1), 145.2 (C-11), 139.3 (C-25), 137.4 (C-12), 136.2 (C-4), 136.0 (C-8), 133.7 (C-22), 130.8 (C-27, 29), 130.7 (C-28), 128.0 (C-26, 30), 127.9 (C-7), 123.4 (C-23), 119.5 (C-3), 118.8 (C-2), 53.9 (C-24), 38.7 (C-9), 37.0 (C-21), 34.8 (C-5), 33.6 (C-15), 29.3 (C-14), 27.8 (C-13), 26.1 (C-10), 24.8 (C-6), 22.1 (C-16, 17), 18.2 (C-18), 16.9 (C-19)。
2.3 N-[1-肉桂基苄基-1H-1, 2, 3-三氮唑-4-亚甲基]-新巴豆瑞士松酰胺2k的合成 2.3.1 肉桂基叠氮(6)的合成将肉桂基溴(197 mg,1 mmol)溶解于8 mL丙酮中,再加入2 mL蒸馏水,混匀,加入叠氮化钠(97 mg,1.5 mmol),室温搅拌反应1 h,TLC检测反应完全,加入30 mL水,二氯甲烷(30 mL×3)萃取,无水硫酸钠干燥,减压浓缩,硅胶柱色谱纯化,洗脱剂为石油醚-醋酸乙酯(100:1),得到无色油状物,收率为90%。
2.3.2 N-[1-肉桂基苄基-1H-1, 2, 3-三氮唑-4-亚甲基]-新巴豆瑞士松酰胺(2k)的合成合成方法同“2.2.2”项2b,无色油状物,收率为76%。HR-MS m/z: 499.343 6 [M+H]+(计算值499.343 7,C32H43N4O)。1H-NMR (600 MHz, CDCl3) δ: 7.60 (1H, s, H-23), 7.35 (2H, dd, J=7.4, 1.7 Hz, H-29, 31), 7.31 (2H, dd, J=8.0, 1.6 Hz, H-28, 32), 7.26 (1H, m, H-30), 6.65 (1H, d, J=15.9 Hz, H-26), 6.30 (1H, dt, J=6.8, 2.2 Hz, H-25), 6.20 (1H, t, J=5.7 Hz, H-N), 5.94 (1H, s, H-2), 5.93 (1H, s, H-3), 5.82 (1H, t, J=7.5 Hz, H-11), 5.08 (2H, d, J=6.9 Hz, H-24), 5.06 (1H, t, J=6.8 Hz, H-7), 4.41 (2H, d, J=5.7 Hz, H-21), 2.46 (2H, m, H-10), 2.35~2.30 (1H, m, H-15), 2.29~2.25 (2H, m, H-13), 2.25~2.22 (2H, m, H-14), 2.21~2.18 (2H, m, H-6), 2.17~2.14 (2H, m, H-9), 2.14~2.12 (2H, m, H-5), 1.71 (3H, s, H-18), 1.64 (3H, s, H-19), 1.00 (3H, s, H-16), 0.99 (3H, s, H-17);13C-NMR (150 MHz, CDCl3) δ: 171.3 (C-20), 145.8 (C-1), 145.2 (C-11), 138.0 (C-12), 135.9 (C-4), 135.5 (C-8), 135.4 (C-27), 135.3 (C-25), 133.6 (C-22), 128.7 (C-29, 31), 128.5 (C-30), 127.8 (C-7), 126.7 (C-28, 32), 122.2 (C-23), 121.6 (C-26), 119.6 (C-3), 118.8 (C-2), 52.4 (C-24), 38.8 (C-9), 37.0 (C-21), 35.2 (C-5), 33.6 (C-15), 29.4 (C-14), 27.5 (C-13), 26.0 (C-10), 24.9 (C-6), 22.1 (C-16, 17), 18.2 (C-18), 16.9 (C-19)。
3 体外抗肿瘤活性评价采用MTT法,以依托泊苷(VP-16)为阳性对照药,测试了目标化合物2a~2k对HeLa、K562和K562/A02 3种肿瘤细胞的体外抗肿瘤活性,实验结果见表 1。
|
|
表 1 目标化合物的体外抗肿瘤活性 Table 1 In vitro antitumor activities of target compounds |
4 讨论
从表 1中可以看出,在HeLa肿瘤细胞株的测试中,化合物2f表现出了良好的抗肿瘤活性,其活性优于阳性对照药依托泊苷,说明衍生物中苄基对位连有氯原子对抗肿瘤活性起到重要作用。而在K562肿瘤细胞株测试中,2e表现出较好的活性,提示衍生物中苄基对位为溴原子取代有助于化合物抗肿瘤活性的提高。值得注意的是,在耐药肿瘤细胞株K562A/02的测试中,化合物2e同样表现出了良好的活性,其抗肿瘤结果与不耐药的K562相当,且活性优于阳性对照药依托泊苷,说明该类化合物具有很好的抗多药耐药性,具有深入的研究价值。
| [1] | Dauhen W G, Thiessen W E, Resnick P R. Cembrene, a 14-membered ring diterpene hydrocarbon[J]. J Am Chem Soc, 1962, 84(10): 2015–2016. DOI:10.1021/ja00869a057 |
| [2] | Kobayashi H, Akiyoshi S. Thunbergene, a macrocyclic diterpene[J]. Bull Chem Soc Jpn, 1962, 35(6): 1044–1045. DOI:10.1246/bcsj.35.1044 |
| [3] | 孙汉董. 二萜化学[M]. 北京: 化学工业出版社, 2012. |
| [4] | El-Sayed K A, Laphookhieo S, Baraka H N, et al. Biocatalytic and semisynthetic optimization of the anti-invasive tobacco (1S, 2E, 4R, 6R, 7E, 11E)-2, 7, 11-cembratriene-4, 6-diol[J]. Bioorg Med Chem, 2008, 16(6): 2886–2893. DOI:10.1016/j.bmc.2007.12.056 |
| [5] | 梁林富, 李玉芬, 刘海利, 等. 短指软珊瑚属次级代谢产物化学成分及其生物活性研究进展[J]. 国际药学研究杂志, 2013, 40(6):643–669. |
| [6] | 李裕林, 李瀛. 西松烷型二萜天然产物全合成最新进展[J]. 化学通报, 1994, 3(1):1–9. |
| [7] | 李国强, 张艳玲, 林文翰. 西松烷二萜类海洋活性成分研究进展[J]. 中国海洋大学学报:自然科学版, 2006, 36(3):370–376. |
| [8] | Wanzola M, Furuta T, Kohno Y, et al. ChemInform abstract:Four new cembrane diterpenes isolated from an okinawan soft coral lobophytum crassum with inhibitory effects on nitric oxide production[J]. Chem Pharm Bull, 2010, 58(9): 1203–1209. DOI:10.1248/cpb.58.1203 |
| [9] | Carvalho J C, Silva M F, Maciel M A, et al. Investigation of anti-inflammatory and antinociceptive activities of trans-dehydrocrotonin, a 19-nor-clerodane diterpene from Croton cajucara. Part 1[J]. Planta Med, 1996, 62(5): 402–404. DOI:10.1055/s-2006-957925 |
| [10] | Lin W Y, Su J H, Lu Y, et al. Cytotoxic and anti-inflammatory cembranoids from the Dongsha atoll soft coral Sarcophyton crassocaule[J]. Bioorg Med Chem, 2010, 18(5): 1936–1941. DOI:10.1016/j.bmc.2010.01.036 |
| [11] | Januar H I, Chasanah E, Motti C A, et al. Cytotoxic cembranes from indonesian specimens of the soft coral Nephthea sp[J]. Mar Drugs, 2010, 8(7): 2142–2152. DOI:10.3390/md8072142 |
| [12] | Yan P C, Deng Z W, Ofwegen L V, et al. Lobophytones O-T, new biscembranoids and cembranoid from soft coral Lobophytum pauciflorum[J]. Mar Drugs, 2010, 8(11): 2837–2848. DOI:10.3390/md8112848 |
| [13] | Su J H, Wen Z H. Bioactive cembrane-bases diterpenoids from the soft coral Sinularia triangular[J]. Mar Drugs, 2011, 9(6): 944–951. |
| [14] | Kao C Y, Su J H, Lu M C, et al. Lobocrassins A-E:new cembrane-type diterpenoids from the soft coral Lobophytum crassum[J]. Mar Drugs, 2011, 9(8): 1319–1331. |
| [15] | Lee C H, Kao C Y, Kao S Y, et al. Terpenoids from the Octocorals Menella sp. (Plexauridae) and Lobophytum crassum (Alcyonacea)[J]. Mar Drugs, 2012, 10(2): 427–438. |
| [16] | Zhao M, Yin J, Jiang W, et al. Cytotoxic and antibacterial cembranoids from a south China sea soft coral, Lobophytum sp.[J]. Mar Drugs, 2013, 11(4): 1162–1172. DOI:10.3390/md11041162 |
| [17] | Liu Z, Cheng W, Liu D, et al. Capnosane-type cembranoids from the soft coral Sarcophyton trocheliophorum with antibacterial effects[J]. Tetrahedron, 2014, 70(16): 8703–8713. |
| [18] | 任晋, 苏亚伦. 西松烷型大环二萜类化合物研究进展[J]. 中草药, 2014, 45(20):2997–3008. |
| [19] | Hou P Y, Zeng Y, Ma B J, et al. A new cytotoxic cembrane diterpene from the roots of Euphorbia pekinensis Rupr[J]. Fitoterapia, 2013, 90(10): 10–13. |
| [20] | El-Mekkawy S, Meselhy M R, Nakamura N, et al. Anti-HIV-1 phorbol esters from the seeds of Croton tiglium[J]. Phytochemistry, 2000, 53(4): 457–464. DOI:10.1016/S0031-9422(99)00556-7 |
| [21] | Wang G H, Huang H C, Su J H, et al. Crassocolides N-P, three cembranoids from the Formosan soft coral Sarcophyton crassocaule[J]. Bioorg Med Chem Lett, 2011, 21(23): 7201–7204. DOI:10.1016/j.bmcl.2011.09.052 |
| [22] | Block S, Caroline S, De Pauw-Gillet M C, et al. ent-Trachyloban-3β-ol, a new cytotoxic diterpene from Croton zambesicus[J]. Planta Med, 2002, 68(7): 647–649. DOI:10.1055/s-2002-32903 |
| [23] | Al-Lihaibi S S, Alarif W M, Abdel-Lateff A, et al. Three new cembranoid-type diterpenes from Red Sea soft coral Sarcophyton glaucum:isolation and antiproliferative activity against HepG2 cells[J]. Eur J Med Chem, 2014, 81(49): 314–322. |
| [24] | Pudhom K, Vilaivan T, Ngamrojanavanich N, et al. Furanocembranoids from the stem bark of Croton oblongifolius[J]. J Nat Prod, 2007, 70(4): 659–661. DOI:10.1021/np060520t |
| [25] | Morales A, Perez P, Mendoza R, et al. Cytotoxic and proapoptotic activity of ent-16β-17α-dihydroxykaurane on human mammary carcinoma cell line MCF-7[J]. Cancer Lett, 2005, 218(1): 109–116. DOI:10.1016/j.canlet.2004.07.009 |
| [26] | Chen Z P, Cai Y, Phillipson J D. Studies on the anti-tumour, anti-bacterial, and wound-healing properties of dragon's blood[J]. Planta Med, 1994, 60(6): 541–545. DOI:10.1055/s-2006-959567 |
| [27] | Kamel H N, Ferreira D, Garcia-Fernandez L F, et al. Cytotoxic diterpenoids from the hybrid soft coral Sinularia maxima×Sinularia polydactyla[J]. J Nat Prod, 2007, 70(8): 1223–1227. DOI:10.1021/np070074p |
| [28] | Hassan H M, Sallam A A, Mohammed R, et al. Semisynthetic analogues of the marine cembranoid sarcophine as prostate and breast cancer migration inhibitors[J]. Bioorg Med Chem, 2011, 19(16): 4928–4934. DOI:10.1016/j.bmc.2011.06.060 |
| [29] | Nermeen A E, Amany K I, Mohamed M R, et al. ChemInform abstract:Cytotoxic cembranoids from the Red Sea soft coral, Sarcophyton auritum[J]. Tetrahedron Lett, 2015, 55(1): 3984–3988. |
| [30] | 江苏新医学院. 中药大辞典[M]. 上海: 上海科学技术出版社, 1975. |
| [31] | 中国科学院中国植物志编辑委员会. 中国植物志[M]. 北京: 北京科学出版社, 1996. |
| [32] | 钟金栋, 李艳平, 李洪梅, 等. 毛叶巴豆的化学成分研究[J]. 天然产物研究与开发, 2013, 25(12):1658–1661. |
| [33] | 邹国安.光叶巴豆、毛叶巴豆化学成分研究[D].北京:中国协和医科大学, 2009. http://www.cnki.com.cn/Article/CJFDTOTAL-ZGYX200911009.htm |
| [34] | Zou G A, Ding G, Su Z H, et al. Lactonecembranoids from Croton laevigatus[J]. J Nat Prod, 2010, 73(4): 792–795. DOI:10.1021/np100044t |
 2017, Vol. 48
2017, Vol. 48


