2. 吉林省中医药科学院植物化学研究所,吉林 长春 130012
2. Institute of Phytochemistry, Jilin Academy of Chinese Medicine Sciences, Changchun 130012, China
蔷薇科(Rosaceae)绣线菊属Spiraea L.植物全世界约有145种,间断分布于欧亚和北美地区[1]。现代药理研究结果表明该属植物具有抗肿瘤[2]、抗血栓[3]、抗血小板聚集[4]、抗疲劳[5]、抗氧化[6]、保肝[7]、神经保护[8]等作用,已从中分离得到二萜生物碱[9-10]、黄酮、木脂素、甾体[11]、糖苷[12]和萜类[13]化合物。柳叶绣线菊Spiraea salicifoliaL.在我国主要分布于黑龙江、吉林、辽宁、内蒙古、河北等省,本课题组前期研究表明,柳叶绣线菊乙醇提取物对佐剂关节炎模型大鼠继发性炎症有明显抑制作用,为进一步寻找其抗类风湿性关节炎活性成分,本研究采用硅胶柱色谱、ODS柱色谱和制备HPLC等手段对其化学成分进行研究,从中分离鉴定了12个化合物,分别为7S, 8R-3, 5-双甲氧基-4′, 7-环氧-8, 5′-新木脂烷-3′, 4, 9, 9′-四醇(7S, 8R-3, 5-dimethoxy-4′, 7-epoxy-8, 5′-neolignan-3′, 4, 9, 9′-tetraol,1)、3β-乙酰基熊果酸甲酯(3β-acetylursolic acid methyl ester,2)、3β-乙酰基齐墩果酸甲酯(3β-acetyloleanolic acid methyl ester,3)、羽扇豆醇(lupeol,4)、β-香树脂醇(β-amyrin,5)、(7R, 8S)-5-甲氧基二氢脱氢双松柏醇[(7R, 8S)-5-methoxydiyhdrodehydroconiferyl alcohol,6]、8-羟基-7′-表松脂醇(8-hydroxy-7′-epipinoresinol,7)、8-羟基松脂醇(8-hydroxypinoresinol,8)、fraxiresinol(9)、(+)-africannal(10)、(+)-南烛木树脂酚[(+)-lyoniresinol,11]、5-甲氧基-(+)-异落叶松脂素[5-methoxy-(+)-isolariciresinol,12]。化合物1为新化合物,命名为柳叶绣线菊新木脂醇;化合物6、7为首次从绣线菊属植物中分离得到,化合物4、5、8~12为首次从该植物中分离得到,化合物2、3为分离过程中产生的熊果酸和齐墩果酸人工产物。
1 仪器与材料Bruker AV-500型核磁共振波谱仪(德国Bruker公司);Bruker micro TOFQ飞行时间质谱仪(德国Bruker公司);JEOL JMS-700型质谱仪(日本电子株式会社);JASCO J-1500型圆二色光谱仪(日本分光株式会社);ShimadzuLC-6AD制备液相色谱输液泵;ShodexRI-102制备液相色谱示差折光检测器(日本昭光电工株式会社)。Shim pack-ODS色谱柱(250 mm×21.2mm,10 μm,日本岛津制作所);Kromasil 100-5-sil色谱柱(250 mm×10 mm,5 μm,瑞典阿克苏诺贝尔公司);Kromasil 100-10-18C色谱柱(250 mm×20 mm,10 μm,瑞典阿克苏诺贝尔公司)。柱色谱用硅胶(200~300目,青岛海洋化工产);柱色谱用ODS(日本Senshu科学株式会社);RP18F254薄层色谱用ODS板(德国默克公司);色谱用甲醇(美国天地公司);水为重蒸馏水;其他试剂均为分析纯。
柳叶绣线菊药材2007年9月采集于吉林省吉林市旺起镇凤凰山,经吉林省中医药科学院徐国经副主任药师鉴定为蔷薇科绣线菊属植物柳叶绣线菊Spiraea salicifolia L.,标本(LYSS-2007-6-1008)存放于吉林省中医药科学院植物化学研究所。
2 提取与分离取柳叶绣线菊根粗粉15 kg,加8倍量60%乙醇回流提取3次,每次2 h,分次滤过,合并滤液,减压回收乙醇得乙醇提取物750 g。取乙醇提取物700 g,加水4 L使溶解,依次用石油醚、氯仿和正丁醇分别振摇提取5次,每次4 L,提取液减压回收,得石油醚部位8 g、氯仿部位30 g、正丁醇部位157 g和水部位450 g。
取氯仿部位25 g,经硅胶柱色谱,以石油醚-氯仿(1∶1),氯仿,氯仿-甲醇(49∶1、19∶1)梯度洗脱得12个分离组分Fr. LYSSC-I(2.56 g)、LYSSC-II(0.17 g)、LYSSC-III(0.37 g)、LYSSC-IV(0.20 g)、LYSSC-V(1.19 g)、LYSSC-VI(1.13 g)、LYSSC-VII(8.95 g)、LYSSC-VIII(0.91 g)、LYSSC-IX(0.64 g)、LYSSC-X(0.30 g)、LYSSC-XI(2.79 g)、LYSSC-XII(2.94 g)。Fr. LYSSC-II经制备HPLC(色谱柱Kromasil 100-5-sil,流动相为正己烷-醋酸乙酯50∶1)得化合物2(14.2 mg)和3(11.6 mg)。Fr. LYSSC-III经制备HPLC(色谱柱Shimpack-ODS,流动相为甲醇)得化合物4(139.5 mg)和5(9.0 mg)。Fr. LYSSC-VI经ODS柱色谱,以甲醇-水(4∶6、6∶4)和甲醇洗脱得6个分离组分Fr. LYSSC-VI-A(0.09 g)、LYSSC-VI-B(0.45 g)、LYSSC-VI-C(0.12 g)、LYSSC-VI-D(0.07 g)、LYSSC-VI-E(0.28 g)、LYSSC-VI-F(0.12 g);Fr. LYSSC-VI-D经制备HPLC(色谱柱Kromasil 100-10-18C,流动相为甲醇-水35∶65)得化合物6(3.6 mg)。Fr. LYSSC-VII经ODS柱色谱,以甲醇-水(4∶6,6∶4)和甲醇洗脱得9个分离组分Fr. LYSSC-VII-1(0.28 g)、LYSSC-VII-2(0.24 g)、LYSSC-VII-3(0.11 g)、LYSSC-VII-4(0.42 g)、LYSSC-VII-5(0.22 g)、LYSSC-VII-6(0.64 g)、LYSSC-VII-7(0.21 g)、LYSSC-VII-8(0.15 g)、LYSSC-VII-9(0.67 g);Fr. LYSSC-VII-5经制备HPLC(色谱柱Kromasil 100-10-18C,流动相为甲醇-水3∶7)得化合物7(31.6 mg);Fr. LYSSC-VII-6经制备HPLC(色谱柱Kromasil 100-10-18C,流动相为甲醇-水3∶7)得化合物8(52.0 mg)和9(6.1 mg)。LYSSC-VIII经ODS柱色谱分离,以甲醇-水(4∶6、7∶3)和甲醇洗脱得8个分离组分LYSSC-VIII-1(0.11 g)、LYSSC-VIII-2(0.02 g)、LYSSC-VIII-3(0.05 g)、LYSSC-VIII-4(0.16 g)、LYSSC-VIII-5(0.03 g)、LYSSC-VIII-6(0.08 g)、LYSSC-VIII-7(0.22 g)、LYSSC-VIII-8(0.19 g);LYSSC-VIII-2经制备HPLC(色谱柱Kromasil 100-10-18C,流动相为甲醇-水3∶7)得化合物10(16.1 mg);LYSSC-VIII-3经制备HPLC(色谱柱Kromasil 100-10-18C,流动相为甲醇-水3∶7)得化合物11(30.6 mg);LYSSC-VIII-4经制备HPLC(色谱柱Kromasil 100-10-18C,流动相为甲醇-水3∶7)得化合物12(30.8 mg)。Fr. LYSSC-X经ODS柱色谱,以甲醇-水(3∶7、5∶5、7∶3)洗脱得3个分离组分Fr. LYSSC-X-1(0.05 g)、LYSSC-X-2(0.08 g)、LYSSC-X-3(0.04 g);Fr. LYSSC-X-3经制备HPLC(色谱柱Shimpack-ODS,流动相为甲醇-水3∶7)得化合物1(12.5 mg)。
3 结构鉴定化合物1:黄色油状物。HR-ESI-MS谱显示准分子离子峰m/z 399.139 5 [M+Na]+,结合13C-NMR谱数据推测分子式为C20H24O7(计算值C20H24O7Na相对分子质量399.142 0)。1H-NMR谱示有对称结构苯环芳香质子单峰信号δ 6.71 (2H, s);2个芳香质子单峰信号δ 6.60 (1H, brs)和6.58 (1H, brs);13C-NMR谱示有12个芳香碳信号,推测结构中存在两个苯环。1H-NMR谱示有1个与氧相连的次甲基质子信号δ 5.50 (1H, d, J=6.2 Hz);1个次甲基质子信号δ 3.45 (1H, m);1组羟甲基质子信号δ 3.85 (1H, dd, J=11.0, 5.2 Hz), 3.76 (1H, dd, J=11.0, 7.6 Hz);1组羟丙基质子信号δ 3.56 (2H, t, J=6.6 Hz), 2.56 (2H, t, J=7.4 Hz), 1.79 (2H, m);2个甲氧基质子信号δ 3.82 (6H, s)。13C-NMR谱示有1个与氧相连的次甲基碳信号δ 88.8;1个次甲基碳信号δ 55.9;1个羟甲基碳信号δ 65.2;1组羟丙基碳信号δ 62.3, 35.8, 32.7。综合解析以上数据,推测化合物1为二氢苯并呋喃木脂素类化合物。HMBC谱显示δH6.58/δC 141.9远程相关信号,推测苯环3′位羟基取代;δH3.85, 3.76与δC 129.7, 88.8, 55.9, δH 5.50, 3.45与δC65.2存在远程相关信号,推测8位羟甲基取代;δH 2.56与δC 117.0, 116.7, δH 6.60, 6.58与δC 32.7有远程相关信号,推测1′位羟丙基取代。根据C-8位化学位移,推测结构中7位和8位为反式构型;CD谱示有287 nm正Cotton效应,推测结构中8位R构型[14]。综合解析以上数据,鉴定化合物1为7S, 8R-3, 5-双甲氧基-4′, 7-环氧-8, 5′-新木脂烷-3′, 4, 9, 9′-四醇(图 1)。该化合物未见文献报道,为新化合物,命名为柳叶绣线菊新木脂醇。化合物1的数据归属见表 1。
|
|
表 1 化合物1的1H-NMR (500MHz, CD3OD)和13C-NMR (125MHz, CD3OD)数据 Table 1 1H-NMR (500 MHz, CD3OD)and 13C-NMR (125 MHz, CD3OD) data of compound 1 |
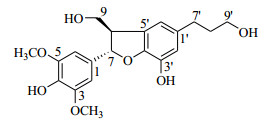
|
图 1 化合物1的结构 Fig.1 Structure of compound 1 |
化合物2:白色粉末(甲醇),Libermann-Burchard反应阳性,EI-MS m/z: 512 [M]+,结合1H-NMR和13C-NMR谱数据推测分子式为C33H52O4。1H-NMR (500 MHz, CDCl3) δ: 5.24 (1H, t, J=3.6 Hz, H-12), 4.50 (1H, dd, J=10.6, 5.6 Hz, H-3), 3.60 (3H, s, COOCH3), 2.04 (3H, s, CH3COO), 1.07 (3H, s, H-27), 0.95 (3H, s, H-25), 0.94 (3H, d, J=4.6 Hz, H-30), 0.89 (3H, d, J=6.8 Hz, H-29), 0.87 (3H, s, H-23), 0.86 (3H, s, H-26), 0.75 (3H, s, H-24);13C-NMR (125 MHz, CDCl3) δ: 178.0 (C-28), 171.0 (CH3COO), 138.2 (C-13), 125.5 (C-12), 81.0 (C-3), 55.4 (C-5), 52.9 (C-18), 51.4 (COOCH3), 48.1 (C-17), 47.5 (C-9), 42.0 (C-14), 39.6 (C-8), 39.1 (C-19), 38.9 (C-20), 38.4 (C-1), 37.7 (C-4), 36.9 (C-10), 36.7 (C-22), 33.0 (C-7), 30.7 (C-21), 28.1 (C-15), 28.1 (C-23), 24.3 (C-16), 23.6 (C-2, 27), 23.3 (C-11), 21.3 (CH3COO), 21.2 (C-30), 18.2 (C-6), 17.1 (C-29), 17.0 (C-24), 16.7 (C-26), 15.5 (C-25)。以上数据与文献报道基本一致[15],故鉴定化合物2为3β-乙酰基熊果酸甲酯。
化合物3:白色粉末(甲醇),Libermann-Burchard反应阳性,EI-MS m/z: 512 [M]+,结合1H-NMR和13C-NMR谱数据推测分子式为C33H52O4。1H-NMR (500 MHz, CDCl3) δ: 5.28 (1H, t, J=3.6 Hz, H-12), 4.49 (1H, dd, J=10.1, 5.8 Hz, H-3), 3.62 (3H, s, COOCH3), 2.86 (1H, dd, J=14.2, 4.4 Hz, H-18), 2.04 (3H, s, CH3COO), 1.13 (3H, s, H-27), 0.93 (3H, s, H-25), 0.92 (3H, s, H-30), 0.90 (3H, s, H-29), 0.86 (3H, s, H-23), 0.85 (3H, s, H-26), 0.72 (3H, s, H-24);13C-NMR (125 MHz, CDCl3) δ: 178.3 (C-28), 171.0 (CH3COO), 143.8 (C-13), 122.3 (C-12), 81.0 (C-3), 55.4 (C-5), 51.5 (COOCH3), 47.6 (C-9), 46.8 (C-17), 45.9 (C-19), 41.7 (C-14), 41.4 (C-18), 39.4 (C-8), 38.2 (C-1), 37.7 (C-4), 37.0 (C-10), 33.9 (C-21), 33.1 (C-29), 32.7 (C-7), 32.4 (C-22), 30.7 (C-20), 28.1 (C-23), 27.5 (C-15), 25.9 (C-27), 23.7 (C-30), 23.6 (C-11), 23.4 (C-2), 23.1 (C-16), 21.3 (CH3COO), 18.27 (C-6), 16.9 (C-24), 16.7 (C-26), 15.4 (C-25)。以上数据与文献报道基本一致[15],故鉴定化合物3为3β-乙酰基齐墩果酸甲酯。
化合物4:白色粉末(甲醇),Libermann-Burchard反应阳性,EI-MS m/z: 426 [M]+,结合1H-NMR和13C-NMR谱数据推测分子式为C30H50O。1H-NMR (500 MHz, CDCl3) δ: 4.69 (1H, d, J=2.3 Hz, H-29a), 4.57 (1H, m, H-29b), 3.19 (1H, m, H-3), 2.38 (1H, ddd, J=11.1, 11.1, 5.8 Hz, H-19), 1.68 (3H, s, H-30), 1.03 (3H, s, H-25), 0.97 (3H, s, H-26), 0.94 (3H, s, H-27), 0.83 (3H, s, H-23), 0.79 (3H, s, H-28), 0.76 (3H, s, H-24);13C-NMR (125 MHz, CDCl3) δ: 151.0 (C-20), 109.3 (C-29), 79.0 (C-3), 55.4 (C-5), 50.5 (C-9), 48.4 (C-18), 48.0 (C-19), 43.0 (C-17), 42.9 (C-14), 40.9 (C-8), 40.0 (C-22), 38.9 (C-4), 38.8 (C-1), 38.1 (C-13), 37.2 (C-10), 35.6 (C-16), 34.3 (C-7), 29.9 (C-21), 28.0 (C-23), 27.5 (C-2), 27.5 (C-15), 25.2 (C-12), 21.0 (C-11), 19.3 (C-30), 18.4 (C-6), 18.0 (C-28), 16.1 (C-25), 16.0 (C-26), 15.4 (C-24), 14.6 (C-27)。以上数据与文献报道基本一致[16],故鉴定化合物4为羽扇豆醇。
化合物5:白色粉末(甲醇),Libermann-Burchard反应阳性,ESI-MS m/z: 426 [M+H]+,结合1H-NMR和13C-NMR谱数据推测分子式为C30H50O。1H-NMR (500 MHz, CDCl3) δ: 5.18 (1H, t, J=3.8 Hz, H-12), 3.22 (1H, brd, H-3), 1.14 (3H, s, H-27), 1.00 (3H, s, H-26), 0.97 (3H, s, H-25), 0.94 (3H, s, H-23), 0.87 (6H, s, H-28, 29), 0.82 (3H, s, H-30), 0.79 (3H, s, H-24);13C-NMR (125 MHz, CDCl3) δ: 145.2 (C-13), 121.8 (C-12), 79.1 (C-3), 55.2 (C-5), 47.7 (C-9), 47.3 (C-18), 46.9 (C-19), 41.8 (C-14), 39.9 (C-8), 38.8 (C-4), 38.6 (C-1), 37.2 (C-22), 37.0 (C-10), 34.8 (C-21), 33.4 (C-29), 32.7 (C-7), 32.5 (C-17), 31.1 (C-20), 28.4 (C-28), 28.1 (C-23), 27.3 (C-2), 27.0 (C-16), 26.2 (C-15), 26.0 (C-27), 23.7 (C-30), 23.6 (C-11), 18.4 (C-6), 16.8 (C-26), 15.6 (C-25), 15.5 (C-24)。以上数据与文献报道基本一致[17],故鉴定化合物5为β-香树脂醇。
化合物6:黄色油状物,ESI-MS m/z: 413 [M+Na]+,结合1H-NMR和13C-NMR谱数据推测分子式为C21H26O7。1H-NMR (500 MHz, CD3OD) δ: 6.73 (2H, s, H-2, 6), 6.68 (2H, s, H-2′, 6′), 5.50 (1H, d, J=6.2 Hz, H-7), 3.87 (3H, s, 3′-OCH3), 3.84 (1H, dd, J=11.0, 5.8 Hz, H-9a), 3.81 (6H, s, 3, 5-OCH3), 3.76 (1H, dd, J=11.1, 7.3 Hz, H-9b), 3.57 (2H, t, J=6.4 Hz, H-9′), 3.47 (1H, dt, J=6.2, 6.0 Hz, H-8), 2.63 (2H, t, J=7.4 Hz, H-7′), 1.82 (2H, m, H-8′);13C-NMR (125 MHz, CD3OD) δ: 149.4 (C-3, 5), 147.6 (C-3′), 145.2 (C-4′), 137.0 (C-1′), 136.5 (C-4), 134.0 (C-1), 129.9 (C-5′), 118.0 (C-6′), 114.2 (C-2′), 104.2 (C-2, 6), 89.1 (C-7), 65.0 (C-9), 62.2 (C-9′), 56.8 (C-8), 56.8 (3, 5-OCH3), 55.6 (3′-CH3), 35.8 (C-8′), 32.9 (C-7′)。以上数据与文献报道基本一致[18],故鉴定化合物6为(7R, 8S)-5-甲氧基二氢脱氢双松柏醇。
化合物7:黄色油状物,ESI-MS m/z: 397 [M+Na]+,结合1H-NMR和13C-NMR谱数据推测分子式为C20H22O7。1H-NMR (500 MHz, CD3OD) δ: 7.04 (1H, d, J=1.7 Hz, H-2), 6.94 (1H, brs, H-2′), 6.85 (1H, dd, J=8.1, 1.7 Hz, H-6), 6.78 (3H, overlap, H-5, 5′, 6′), 5.17 (1H, d, J=6.4 Hz, H-7′), 4.39 (1H, s, H-7), 4.19 (1H, d, J=9.1 Hz, H-9a), 3.90 (1H, dd, J=9.2, 9.1 Hz, H-9′a), 3.86 (3H, s, 3-OCH3), 3.86 (3H, s, 3′-OCH3), 3.61 (1H, d, J=9.2 Hz, H-9b), 3.22 (1H, dd, J=9.2, 9.1 Hz, H-9′b), 3.09 (1H, ddd, J=9.1, 9.1, 6.4 Hz, H-8′);13C-NMR (125 MHz, CD3OD) δ: 148.9 (C-3′), 148.7 (C-3), 147.5 (C-4), 146.7 (C-4′), 131.1 (C-1′), 129.0 (C-1), 121.6 (C-6), 119.3 (C-6′), 116.1 (C-5′), 115.7 (C-5), 112.9 (C-2), 110.5 (C-2′), 91.6 (C-8), 90.7 (C-7), 82.8 (C-7′), 76.8 (C-9), 69.0 (C-9′), 58.7 (C-8′), 56.46 (3-OCH3), 56.42 (3′-OCH3)。以上数据与文献报道基本一致[19] ,故鉴定化合物7为8-羟基-7′-表松脂醇。
化合物8:黄色油状物,ESI-MS m/z: 397 [M+Na]+,结合1H-NMR和13C-NMR谱数据推测分子式为C20H22O7。1H-NMR (500 MHz, CD3OD) δ: 7.05 (1H, s, H-2′), 7.04 (1H, d, J=1.4 Hz, H-2), 6.86 (1H, dd, J=8.5, 1.8 Hz, H-6′), 6.85 (1H, dd, J=8.6, 1.8 Hz, H-6), 6.79 (1H, d, J=8.1 Hz, H-5), 6.79 (1H, d, J=8.1 Hz, H-5′), 4.83 (1H, d, J=5.4 Hz, H-7′), 4.67 (1H, s, H-7), 4.45 (1H, dd, J=9.2, 8.6 Hz, H-9′a), 4.03 (1H, d, J=9.3 Hz, H-9a), 3.86 (1H, d, J=9.3 Hz, H-9b), 3.86 (3H, s, 3-OCH3), 3.85 (3H, s, 3′-OCH3), 3.75 (1H, dd, J=9.2, 6.2 Hz, H-9′b), 3.04 (1H, ddd, J=8.6, 6.2, 5.4 Hz, H-8′);13C-NMR (125 MHz, CD3OD) δ: 149.1 (C-3′), 148.7 (C-3), 147.5 (C-4), 147.4 (C-4′), 133.6 (C-1′), 129.1 (C-1), 121.5 (C-6), 120.5 (C-6′), 116.1 (C-5′), 115.7 (C-5), 112.8 (C-2), 111.4 (C-2′), 92.8 (C-8), 89.2 (C-7), 87.8 (C-7′), 76.1 (C-9), 72.0 (C-9′), 62.4 (C-8′), 56.4 (3, 3′-OCH3)。以上数据与文献报道基本一致[20],故鉴定化合物8为8-羟基松脂醇。
化合物9:黄色油状物,ESI-MS m/z: 427 [M+Na]+,结合1H-NMR和13C-NMR谱数据推测分子式为C21H24O8。1H-NMR (500 MHz, CD3OD) δ: 7.05 (1H, d, J=1.8 Hz, H-2′), 6.87 (1H, dd, J=8.1, 1.9 Hz, H-6′), 6.78 (1H, d, J=8.7 Hz, H-5′), 6.72 (2H, s, H-2, 6), 4.85 (1H, overlap, H-7′), 4.69 (1H, s, H-7), 4.47 (1H, dd, J=9.2, 7.8 Hz, H-9′a), 4.07 (1H, d, J=9.4 Hz, H-9a), 3.89 (1H, d, J=9.4 Hz, H-9b), 3.86 (9H, s, 3, 3′, 5-OCH3), 3.77 (1H, dd, J=9.2, 6.2 Hz, H-9′b), 3.05 (1H, ddd, J=7.8, 6.2, 5.9 Hz, H-8′);13C-NMR (125 MHz, CD3OD) δ: 149.2 (C-3′), 149.0 (C-3, 5), 147.5 (C-4′), 136.6 (C-4), 133.6 (C-1′), 128.2 (C-1), 120.5 (C-6′), 116.1 (C-5′), 111.4 (C-2′), 106.3 (C-2, 6), 92.9 (C-8), 89.4 (C-7), 87.8 (C-7′), 76.1 (C-9), 72.0 (C-9′), 62.4 (C-8′), 56.8 (3, 5-OCH3), 56.4 (3′-OCH3)。以上数据与文献报道基本一致[21],故鉴定化合物9为fraxiresinol。
化合物10:白色粉末,[α]D25 +180.6°(c 0.22, CH3OH),ESI-MS m/z: 375 [M+H]+,结合13C-NMR谱数据推测分子式为C20H22O7。1H-NMR (500 MHz, CD3OD) δ: 6.76 (1H, d, J=8.0 Hz, H-5), 6.71 (1H, s, H-2′), 6.69 (1H, brs, H-2), 6.65 (1H, dd, J=8.1, 1.6 Hz, H-6), 6.26 (1H, s, H-5′), 5.16 (1H, s, H-9′), 3.90 (1H, d, J=11.8 Hz, H-7), 3.82 (3H, s, 3′-OCH3), 3.78 (1H, s, 3-OCH3), 3.74 (1H, d, J=4.8 Hz, H-9a), 3.72 (1H, d, J=2.6 Hz, H-9b), 3.30 (1H, d, J=15.6 Hz, H-7′a), 2.84 (1H, d, J=16.6 Hz, H-7′b), 2.57 (1H, m, H-8);13C-NMR (125 MHz, CD3OD) δ: 149.3 (C-3), 147.8 (C-3′), 146.4 (C-4), 145.4 (C-4′), 137.4 (C-1), 133.4 (C-6′), 127.2 (C-1′), 122.5 (C-6), 116.8 (C-5′), 116.2 (C-5), 114.0 (C-2), 113.0 (C-2′), 104.3 (C-9′), 79.8 (C-8′), 71.2 (C-9), 56.5 (3-OCH3), 56.4 (3′-OCH3), 48.5 (C-8), 45.4 (C-7), 37.0 (C-7′)。以上数据与文献报道基本一致[22],故鉴定化合物10为(+)-africannal。
化合物11:白色粉末,[α]25D +62.90° (c 0.28, CH3OH),ESI-MS m/z: 421 [M+H]+,结合13C-NMR谱数据推测分子式为C22H28O8。1H-NMR (500 MHz, CD3OD) δ: 6.58 (1H, s, H-2′), 6.38 (2H, s, H-2, 6), 4.31 (1H, d, J=5.6 Hz, H-7), 3.86 (3H, s, 3′-OCH3), 3.74 (6H, s, 3, 5-OCH3), 3.59 (1H, dd, J=10.8, 5.1 Hz, H-9′a), 3.50 (3H, m, H-9, 9′b), 3.38 (3H, s, 5′-OCH3), 2.70 (1H, dd, J=15.0, 4.8 Hz, H-7′a), 2.57 (1H, dd, J=14.8, 11.4 Hz, H-7′b), 1.97 (1H, m, H-8), 1.63 (1H, m, H-8′);13C-NMR (125 MHz, CD3OD) δ: 149.0 (C-3, 5), 148.7 (C-3′), 147.7 (C-5′), 139.3 (C-4′), 138.9 (C-1), 134.6 (C-4), 130.2 (C-1′), 126.3 (C-6′), 107.8 (C-2′), 107.0 (C-2, 6), 66.8 (C-9′), 64.3 (C-9), 60.2 (5′-OCH3), 56.8 (3, 5-OCH3), 56.6 (3′-OCH3), 49.0 (C-8), 42.3 (C-7), 40.9 (C-8′), 33.6 (C-7′)。以上数据与文献报道基本一致[23],故鉴定化合物11为(+)-南烛木树脂酚。
化合物12:白色粉末,[α]D25 +38.20° (c 0.25, CH3OH),ESI-MS m/z: 391 [M+H]+,结合13C-NMR谱数据推测分子式为C21H26O7。1H-NMR (500 MHz, CD3OD) δ: 6.66 (1H, s, H-2′), 6.43 (2H, s, H-2, 6), 6.21 (1H, s, H-5′), 3.81 (3H, s, 3′-OCH3), 3.78 (1H, d, J=10.4 Hz, H-7), 3.78 (6H, s, 3, 5-OCH3), 3.69 (3H, m, H-9′, 9a), 3.41 (1H, dd, J=11.2, 4.0 Hz, H-9b), 2.78 (2H, d, J=7.7 Hz, H-7′), 2.01 (1H, m, H-8′), 1.79 (1H, m, H-8);13C-NMR (125 MHz, CD3OD) δ: 149.3 (C-3, 5), 147.3 (C-3′), 145.4 (C-4′), 137.8 (C-1), 135.2 (C-4), 134.0 (C-6′), 129.0 (C-1′), 117.3 (C-5′), 112.5 (C-2′), 107.8 (C-2, 6), 66.0 (C-9′), 62.3 (C-9), 56.8 (3, 5-OCH3), 56.4 (3′-OCH3), 48.5 (C-7), 47.9 (C-8), 40.0 (C-8′), 33.6 (C-7′)。以上数据与文献报道基本一致[24],故鉴定化合物12为5-甲氧基-(+)-异落叶松脂素。
| [1] | 谢华辉, 杨莉莉, 包志毅. 绣线菊属植物资源及其在园林中的应用前景[J]. 林业科学 , 2006, 42 (7) :104–112. |
| [2] | Yan C, Huang L, Liu H C, et al. Spiramine derivatives induce apoptosis of Bax−/−/Bak−/−cell and cancer cells[J]. Bioorg Med Chem Lett , 2014, 24 (8) :1884–1888. DOI:10.1016/j.bmcl.2014.03.019 |
| [3] | Shen Z Q, Chen Z H, Li L, et al. Antiplatelet and antithrombotic effects of the diterpene spiramine Q from Spiraea japonica var. incise[J]. Planta Med , 2000, 66 (3) :287–289. DOI:10.1055/s-2000-8571 |
| [4] | 李玲, 李民, 沈月毛, 等. 华北绣线菊二萜生物碱抗血小板聚集活性研究[J]. 天然产物研究与开发 , 2002, 14 (3) :7–10. |
| [5] | 韩咏梅, 刘红微, 赵丽芹. 土庄绣线菊水浸液对小鼠抗疲劳作用的试验研究[J]. 内蒙古农业大学学报:自然科学版 , 2014, 35 (2) :9–12. |
| [6] | 孔祥密, 王金梅, 魏金凤, 等. 疏毛绣线菊的抗氧化活性研究[J]. 天然产物研究与开发 , 2014, 26 (8) :1308–1310. |
| [7] | 刘红微, 赵丽芹, 张田田, 等. 土庄绣线菊水浸液对小鼠急性酒精肝损伤保护作用的研究[J]. 中国畜牧兽医 , 2012, 39 (4) :166–169. |
| [8] | Li L, Nie J L, Shen Z Q, et al. Neroroprotective effects in gerbils of spiramine T from Spiraea japonica var. acuta[J]. Planta Med , 2001, 67 (2) :142–145. DOI:10.1055/s-2001-11510 |
| [9] | Zuo G Y, He H P, Hong X, et al. New spiramines from Spiraea japonica var. ovalifolia[J]. Heterocycles , 2001, 55 (3) :487–493. DOI:10.3987/COM-00-9105 |
| [10] | Liu H Y, Ni W, Chen C X, et al. Two new diterpenoid lactams from Spiraea japonica var. ovalifolia[J]. Helv Chim Acta , 2009, 92 (6) :1198–1202. DOI:10.1002/hlca.v92:6 |
| [11] | Wu T S, Hwang C C, Kuo P C, et al. New neolignans from Spiraea formosana[J]. Chem Pharm Bull , 2004, 52 (10) :1227–1230. DOI:10.1248/cpb.52.1227 |
| [12] | Choudhary M I, Naheed N, Abbaskhan A, et al. Hemiterpene glucosides and other constituents from Spiraea canescens[J]. Phytochemistry , 2009, 70 (11/12) :1467–1473. |
| [13] | Hao X J, Nie J L. Diterpenes from Spiraea japonica[J]. Phytochemistry , 1998, 48 (7) :1213–1215. DOI:10.1016/S0031-9422(97)00791-7 |
| [14] | Wang Y H, Sun Q Y, Yang F M, et al. Neolignans and caffeoyl derivatives from Selaginella moellendorffii[J]. Helv Chim Acta , 2010, 93 (12) :2467–2477. DOI:10.1002/hlca.v93.12 |
| [15] | Niesen A, Barthel A, Kluge R, et al. Antitumoractive endoperoxides from Triterpenes[J]. Archiv Der Pharmazie , 2009, 342 (10) :569–576. DOI:10.1002/ardp.v342:10 |
| [16] | Zhang N, Li N, Sun Y N, et al. Diacylglycerol compounds from barks of Betula platyphylla with inhibitory activity against acyltransferase[J]. Chin Herb Med , 2014, 6 (2) :164–167. DOI:10.1016/S1674-6384(14)60026-5 |
| [17] | Zhang T, Ye Q, Feng C, et al. Chemical study on Gladiolus gandavensis[J]. Chin J Appl Environ Biol , 2007, 13 (5) :635–640. |
| [18] | Meng J X, Jiang T, Bhatti H A, et al. Synthesis of dihydrodehydrodiconiferyl alcohol: the revised structure of lawsonicin[J]. Org Biomol Chem , 2010, 8 (1) :107–113. DOI:10.1039/B918179B |
| [19] | Tsukamoto H, Hisada S, Nishibe S. Lignans from bark of the Olea plants. II[J]. Chem Pharm Bull , 1985, 33 (3) :1232–1241. DOI:10.1248/cpb.33.1232 |
| [20] | Yeo H, Chin Y W, Park S Y, et al. Lignans of Rosa multiflora roots[J]. Arch Pharm Res , 2004, 27 (3) :287–290. DOI:10.1007/BF02980061 |
| [21] | Tsukamoto H, Hisada S, Nishide S. Lignans from bark of Fraxinus mandshurica var. japonica and F. japonica[J]. Chem Pharm Bull , 1984, 32 (11) :4482–4489. DOI:10.1248/cpb.32.4482 |
| [22] | Viviers P M, Ferreira D, Roux G D. (+)-Africanal, a new lignan of the aryltetrahydronaphthalene class[J]. Tetrahedron Lett , 1979, 20 (39) :3773–3776. DOI:10.1016/S0040-4039(01)95521-2 |
| [23] | Ouyang M A, Wein Y S, Su R K, et al. Rhusemialins A-C, new cycloligan esters from the roots of Rhus javanica var. roxburghiana[J]. Chem Pharm Bull , 2007, 55 (5) :804–807. DOI:10.1248/cpb.55.804 |
| [24] | Zhang Z, Li C J, Koike K, et al. Gaultherins A and B, two lignans from Gaultheria yunnanensis[J]. Phytochemistry , 1999, 51 (3) :469–472. DOI:10.1016/S0031-9422(99)00029-1 |
 2016, Vol. 47
2016, Vol. 47


