槟榔是棕榈科(Palmae)植物槟榔Areca catechu Linn. 的干燥成熟种子,位居我国四大南药(槟榔、益智、砂仁、巴戟)之首,是54种棕榈科植物中唯一含生物碱的植物。槟榔嚼块是仅次于烟草、酒精和咖啡因的世界第4种被广泛使用的嗜好品。嚼食槟榔与口腔癌、肥胖、糖尿病、高血压、高脂血症、肝硬化和肝细胞癌等相关[1-2],这与槟榔所含的主要生物碱成分可诱导DNA分子单链断裂,姊妹染色单体交换频率与基因突变密切相关[3-4]。槟榔碱是槟榔所含的主要生物碱,占干质量的0.1%~0.5%[5]。
近年来国内外众多学者对槟榔碱药理、毒理等方面进行了深入研究,发现槟榔碱对口腔黏膜[6]、神经系统[7]、内分泌系统[8-10]、生殖细胞[11]等均存在一定毒副作用。此外,槟榔碱还能导致广泛的肝脏代谢影响,诱导肝脏代谢酶活性升高[12-14]。
CYP2B6是人体一个重要的药物代谢酶,参与了许多药物、毒物及内源性神经递质的代谢清除。大鼠与人CYP2B6基因高度同源的是CYP2B1/2[15]。研究表明,大鼠、小鼠肝脏中CYP2B活性的升高,与肝纤维化及肝肿瘤形成有密切关系[16-17]。前期研究表明,氢溴酸槟榔碱在大鼠体内代谢产生了4个I相代谢物和2个II相代谢物[18],且氢溴酸槟榔碱短期给药诱导了大鼠肝脏CYP2B1/2活性[14]。但氢溴酸槟榔碱体内对大鼠肝脏CYP2B1/2的调控机制目前尚不清楚。本实验研究氢溴酸槟榔碱ig给药对大鼠肝脏CYP2B1/2表达的影响及其机制,为阐明槟榔碱的毒性及药物相互作用提供参考。
1 材料 1.1 实验动物SPF级6周龄Wistar雄性大鼠,体质量200~250 g,购自湖北省实验动物研究中心,许可证号SCXK(鄂)2015-0018。
1.2 药品与试剂氢溴酸槟榔碱(Sigma,美国);安非他酮、羟基安非他酮(Cayman Chemical,美国);TRIzol试剂(Invitrogen,美国);PVDF膜(millipore,美国);蛋白酶抑制剂cocktail(Roche,瑞典);RIPA裂解液、超敏ECL化学发光试剂盒(碧云天生物技术公司,南京);BCA蛋白测定试剂盒(Thermo Scientific,美国);小鼠抗β-actin单克隆抗体、兔抗组成型雄甾烷受体(CAR)单克隆抗体(Santa Cruz,美国);小鼠抗CYP2B1/2单克隆抗体(Abcam,美国);兔抗Lamin B抗体(武汉博士德生物工程有限公司);山羊抗小鼠/兔辣根过氧化物酶标记二抗(KPL,美国);定量PCR引物(Sunny,上海);逆转录试剂盒及SYBR qPCR Mix(Toyobo,日本);NE-PER核蛋白提取试剂盒(Thermo Fisher,美国)。
1.3 主要仪器8040型高效液相色谱质谱联用仪(岛津,日本);组织匀浆器(IKA,德国),核酸蛋白测定仪(eppendorf,德国);CFX ConnectTM实时荧光定量PCR仪(Bio-Rad,美国);DYY-6C型电泳仪、DYC-40A型垂直电泳槽及DYC-40A型电转仪(北京六一生物科技有限公司)。
2 方法 2.1 实验动物分组与给药雄性Wistar大鼠随机分为氢溴酸槟榔碱(溶解于生理盐水中)低、中、高剂量[4、20、100 mg/(kg·d)]组和对照组(等体积生理盐水),每组6只。饲养温度保持在20~25 ℃,相对湿度在40%~70%,自由取食、进水,昼夜交替时间为12 h/12 h。给药前大鼠禁食12 h,每天于同一时间ig给药(给药体积小于2.0 mL),连续给药7 d。末次给药后1 h颈椎脱臼处死大鼠,取肝脏组织,用预冷生理盐水冲洗后擦干,液氮中保存备用。
2.2 荧光定量PCR检测肝脏组织CYP2B1 mRNA的表达每只大鼠取肝组织约100 mg,按TRIzol试剂盒说明书分别提取总RNA,用0.7%的琼脂糖凝胶电泳检测总RNA的完整性,用分光光度法检测260 nm和280 nm处吸光度比值检测总RNA的纯度。取2 μg RNA按逆转录试剂盒说明书逆转录成cDNA,cNDA再经实时荧光定量PCR扩增检测CYP2B1 mRNA的表达量。定量PCR引物序列如下,大鼠GAPDH:正向5’-AGGGCTGCCTTCTCTTGTGAC-3’,反向5’-TGGGTAGAATCATACTGGA- ACATGTAG-3’;大鼠CYP2B1:正向5’-AAGCACAGGGCCACCTTA- GAC-3’,反向5’-AAGCACAGGGCCACCTTAG- AC-3’。定量PCR实验条件为94 ℃变性15 s,58.4 ℃退火30 s,72 ℃延伸30 s,38个循环,反应体系为20 μL。基因表达水平采用2−ΔΔCt法计算[19]。
2.3 Western blotting检测肝脏组织CYP2B和细胞内总CAR蛋白的表达取适量(约100 mg)肝组织加入800 μL裂解液中冰浴匀浆,离心取上清,用BCA试剂盒检测蛋白量。取60 μg蛋白样品经SDS-PAGE(10%分离胶与5%浓缩胶)后转移到PVDF膜上,经5%脱脂奶粉封闭1.5 h后与一抗(小鼠抗β-actin单克隆抗体,小鼠抗CYP2B1/2单克隆抗体,兔抗CAR单克隆抗体,用一抗稀释液按1∶1 000稀释)4 ℃孵育过夜。用TBST洗涤后加二抗(用脱脂牛奶按1∶5 000稀释)37 ℃温孵1 h,再用TBST洗涤,加超敏 ECL化学发光液,经显影、定影后,根据扫描条带的大小与强度分析各蛋白的表达情况。
2.4 Western blotting检测细胞核内CAR蛋白的表达取20 mg大鼠肝组织,用PBS洗涤后,500×g离心5 min,弃上清,按照核蛋白提取试剂盒说明书进行如下操作:加入预冷的CER I 200 μL,匀浆,剧烈涡旋15 s,于冰上静置10 min,然后加入预冷的CER II 11 μL,涡旋5 s,冰上静置1 min,再涡旋5 s,16 000×g离心5 min,弃上清,沉淀用预冷的NER 100 μL溶解。剧烈涡旋15 s,冰上静置10 min,再涡旋15 s,静置10 min,如此40 min(4次)。16 000×g离心10 min取上清,即为核蛋白溶液。用BCA试剂盒检测蛋白的量,取60 μg蛋白样品经SDS-PAGE(10%分离胶与5%浓缩胶)后转移到PVDF膜上,用5%脱脂奶粉封闭1.5 h后与一抗(兔抗Lamin B抗体,用一抗稀释液按1∶400稀释;兔抗CAR多克隆抗体,用一抗稀释液按1∶1 000稀释)4 ℃ 孵育过夜。用TBST洗涤后加二抗(用脱脂牛奶按1∶5 000稀释)37 ℃温孵1 h,再用TBST洗涤,加超敏ECL化学发光液,经显影、定影后,根据扫描条带的大小与强度分析核内CAR的表达情况。
2.5 LC-MS/MS法检测CYP2B 1/2活性采用CaCl2沉淀低速离心法分别制备每只大鼠的肝微粒体(RLM),并用BCA法测定RLM中蛋白量。以安非他酮的羟基化作用表示RLM中CYP2B1/2活性(即每毫克微粒体蛋白每分钟催化探针底物产生的代谢物量)[18]。
2.6 统计学分析采用ANOVA统计学软件,数据以x±s表示,组间差异采用t检验。
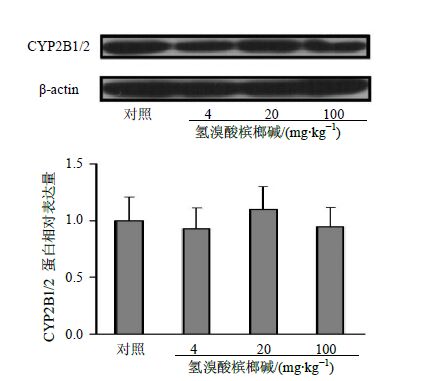
3 结果 3.1 对大鼠肝脏CYP2B表达与活性的影响连续给大鼠ig 4、20、100 mg/kg氢溴酸槟榔碱7 d后,定量荧光PCR检测大鼠肝脏CYP2B1 mRNA的表达量,结果见图 1,Western blotting检测大鼠肝脏CYP2B1/2的蛋白表达量结果见图 2。结果表明,与对照组相比,给药后肝脏CYP2B1 mRNA的表达量有剂量依赖性上调趋势(P<0.05);对大鼠肝脏CYP2B1/2的表达量无明显影响(P>0.05);对大鼠肝脏CYP2B1/2活性的诱导作用随剂量增大而减弱,对照组及氢溴酸槟榔碱低、中、高剂量组CYP2B的相对活性分别为(100.0±25.7)%、(169.5±23.8)%(P<0.01)、(151.4±11.2)%(P<0.01)、(121.6±12.3)%(P>0.05)。

|
与对照组比较:P<0.05 P < 0.05 vs control group 图 1 氢溴酸槟榔碱对大鼠肝脏CYP2B1 mRNA表达的影响(x±s,n = 6) Fig.1 Effect of AH on CYP2B1 mRNA expression in liver tissue of rats (x±s,n = 6) |
|
图 2 氢溴酸槟榔碱对大鼠肝脏CYP2B蛋白表达的影响(x±s,n = 6) Fig.2 Effect of AH on CYP2B protein expression in liver tissue of rats (x±s,n = 6) |
3.2 对大鼠肝脏总CAR及核内CAR蛋白表达水平的影响
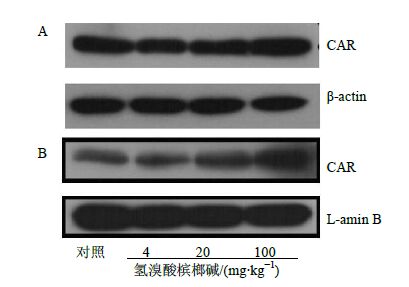
大鼠肝脏细胞内总CAR与核内CAR的蛋白表达量结果见图 3。结果表明,氢溴酸槟榔碱给药后,胞内总CAR蛋白表达量较对照组几乎无变化;核内CAR的蛋白表达量呈明显的上调趋势(P<0.05)。
|
图 3 氢溴酸槟榔碱对肝细胞总CAR (A) 及肝细胞核内CAR(B) 蛋白表达的影响(x±s,n = 6) Fig.3 Effect of AH on protein expression of hepatic total CAR (A) and hepatic cell intranucleus CAR (B) (x±s,n = 6) |
4 讨论
CYP2B6是重要的外源性毒物代谢酶之一,参与代谢杀虫剂(如甲氧滴滴涕)、前致癌物(如黄曲霉毒素B1、烟草中的亚硝胺类物质),以及抗肿瘤药物(如环磷酰胺、异环磷酰胺)的活化[20],其活性可以被许多外源性物质诱导[21-26]。外源物对大鼠肝脏CYP2B1/2和小鼠肝脏CYP2B9/10的转录激活是通过CAR的核移位实现的[27-28],CAR受体由胞浆向核内转移被认为是诱导CYP2B转录的起始步骤[29],CAR在核内与维甲酸X受体RXR形成异二聚体CAR-RXR,然后异二聚体结合到CYP2B基因上游的苯巴比妥反应增强原件PBREM上,启动CYP2B基因的转录[30]。本研究表明,给大鼠连续ig氢溴酸槟榔碱(4、20、100 mg/kg)7 d后,肝脏中CYP2B1 mRNA表达水平与CYP2B1/2活性上调,肝细胞核内CAR蛋白量增加,且肝脏总CAR蛋白量不变,结果表明,氢溴酸槟榔碱对大鼠肝脏CYP2B的诱导作用类似于苯巴比妥及其类似物,也是通过CAR的核移位实现的。
本研究还发现,氢溴酸槟榔碱对大鼠肝脏CYP2B mRNA表达及酶活的诱导作用存在相反的剂量关系,即CYP2B1 mRNA的表达量随氢溴酸槟榔碱的给药剂量增加而增加,而酶活性虽被诱导,但却随给药剂量增加而减弱。另外,氢溴酸槟榔碱给药对肝脏CYP2B1/2蛋白表达量无明显影响,提示槟榔碱对肝脏CYP2B1/2的调控可能存在翻译后修饰和/或RNA转运和/或mRNA降解,具体是何种原因导致的,将在接下来的实验中做进一步探究。对于CYP2B酶活上调而蛋白的量无变化,可能是槟榔碱增加了底物与CYP2B的亲和力。
本研究表明,低剂量(4 mg/kg)氢溴酸槟榔碱是大鼠肝脏CYP2B活性的诱导剂,提示槟榔咀嚼嗜好者在接受CYP2B底物治疗时,存在一定的代谢相互作用风险。
| [1] | Guh J Y, Chuang L Y, Chen H C. Betel-quid use is associated with the risk of the metabolic syndrome in adults[J]. Am J Clin Nutr , 2006, 83 (6) :1313–1320. |
| [2] | Wu P F, Chiang T A, Chen M T, et al. A characterization of the antioxidant enzyme activity and reproductive toxicity in male rats following sub-chronic exposure to areca nut extracts[J]. J Hazard Mater , 2010, 178 (1/3) :541–546. |
| [3] | Jayant K, Balakrishnan V, Sanghvi L D, et al. Quantification of the role of smoking and chewing habits in oral, pharynx, and esophageal cancer[J]. Br J Cancer , 1977, 35 (2) :232–235. DOI:10.1038/bjc.1977.31 |
| [4] | 季宇彬, 李连闯, 于蕾. 槟榔碱对骨髓细胞内DNA的影响[J]. 中草药 , 2007, 38 (4) :573–575. |
| [5] | 赵云霞, 于蕾, 季宁彬. 槟榔碱的毒理研究进展[J]. 药品评价 , 2006, 3 (6) :457–458. |
| [6] | 李忠海, 郑锦星, 袁列江, 等. 槟榔在口腔黏膜下纤维性变发病机制中的作用[J]. 中南林学院学报 , 2006, 26 (6) :145–149. |
| [7] | Shih Y T, Chen P S, Wu C H, et al. Arecoline, a major alkaloid of the areca nut, causes neurotoxicity through enhancement of oxidative stress and suppression of the anti-oxidant protective system[J]. Free Radic Biol Med , 2010, 49 (10) :1471–1479. DOI:10.1016/j.freeradbiomed.2010.07.017 |
| [8] | Wang S W, Hwang G S, Chen T J, et al. Effects of arecoline on testosterone release in rats[J]. Am J Physiol Endocrinol Metab , 2008, 295 (2) :E497–E504. DOI:10.1152/ajpendo.00045.2008 |
| [9] | Calogero A E, Kamilars T C, Gomez M T, et al. The muscarinic cholinergic agonist arecoline stimulates the rat hypothalamic-pituitary-adrenal axis through a centrally-mediated corticotrophin-releasing hormone dependent mechanism[J]. Endocrinology , 1989 (125) :2445–2453. |
| [10] | Lim D Y, Kim I S. Arecoline inhibits catecholamine release from perfused rat adrenal gland[J]. Acta Pharmacol Sin , 2006, 27 (1) :71–79. DOI:10.1111/aphs.2006.27.issue-1 |
| [11] | Liu S T, Young G C, Lee Y C, et al. A preliminary report on the toxicity of arecoline on early pregnancy in mice[J]. Food Chem Toxicol , 2011, 49 (1) :144–148. DOI:10.1016/j.fct.2010.10.009 |
| [12] | Singh A, Rao A R. Effects of arecoline on phase I and phase Ⅱ drug metabolizing system enzymes, sulfhydryl content and lipid peroxidation in mouse liver[J]. Biochem Mol Biol Int , 1993, 30 (4) :763–772. |
| [13] | Singh A, Singh S P, Bamezai R. Direct and transloctational effect of arecoline alkaloid on the clocimum oil-modulated hepatic drug metabolizing enzymes in mice[J]. Food Chem Toxicol , 2000, 38 (7) :627–635. DOI:10.1016/S0278-6915(00)00045-4 |
| [14] | Xiao R M, Wang J J, Chen J Y, et al. Effects of arecoline on hepatic cytochrome P450 activity and oxidative stress[J]. J Toxicol Sci , 2014, 39 (4) :609–614. DOI:10.2131/jts.39.609 |
| [15] | 张庆柱. 分子药理学[M]. 北京: 高等教育出版社, 2007 . |
| [16] | Deguchi Y, Yamada T, Hirose Y, et al. Mode of action analysis for the synthetic pyrethroid metofluthrin-induced rat liver tumors:evidence for hepatic CYP2B induction and hepatocyte proliferation[J]. Toxicol Sci , 2009, 108 (1) :69–80. DOI:10.1093/toxsci/kfp006 |
| [17] | Lo W S, Lim Y P, Chen C C, et al. A dual function of the furanocoumarin chalepensin in inhibiting Cyp2a and inducing Cyp2b in mice:the protein stabilization and receptor-mediated activation[J]. Arch Toxicol , 2012, 86 (12) :1927–1938. DOI:10.1007/s00204-012-0902-7 |
| [18] | Zhu M M, Chen H X, Han F M, et al. Analysis of arecoline in rat urine and identification of its metabolites by liquid chromatography-tandem mass spectrometry[J]. Chromatographia , 2006, 64 (11/12) :705–708. |
| [19] | Livak K J, Schmittgen T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method[J]. Methods , 2001, 25 (4) :402–408. DOI:10.1006/meth.2001.1262 |
| [20] | Turpeinen M, Raunio H, Pelkonen O. The functional role of CYP2B6 in human drug metabolism:substrates and inhibitors in vitro, in vivo and in silico[J]. Curr Drug Metab , 2006, 7 (7) :705–714. DOI:10.2174/138920006778520633 |
| [21] | Swales K, Negishi M. CAR, driving into the future[J]. Mol Endocrinol , 2004, 18 (7) :1589–1598. DOI:10.1210/me.2003-0397 |
| [22] | Yamamoto Y, Moore R, Goldsworthy T L, et al. The orphan nuclear receptor constitutive active/androstane receptor is essential for liver tumor promotion by phenobarbital in mice[J]. Cancer Res , 2004, 64 (20) :7197–7200. DOI:10.1158/0008-5472.CAN-04-1459 |
| [23] | Pustylnyak V O, Lebedev A N, Gulyaeva L F, et al. Comparative study of CYP2B induction in the liver of rats and mice by different compounds[J]. Life Sci , 2007, 80 (4) :324–328. DOI:10.1016/j.lfs.2006.09.015 |
| [24] | Pustylnyak V, Pivovarova E, Slynko N, et al. Species-specific induction of CYP2B by 2,4,6-tryphenyldioxane-1,3(TPD)[J]. Life Sci , 2009, 85 (23-26) :815–821. DOI:10.1016/j.lfs.2009.10.015 |
| [25] | Pustylnyak V, Kazakova Y, Yarushkin A, et al. Effect of several analogs of 2,4,6-triphenyldioxane-1,3 on CYP2B induction in mouse liver[J]. Chem Biol Interact , 2011, 194 (2/3) :134–138. |
| [26] | Catania J R, McGarrigle B P, Rittenhouse-Olson K, et al. Induction of CYP2B and CYP2E1 in precision-cut rat liver slices cultured in defined medium[J]. Toxicol Vitro , 2007, 21 (1) :109–115. DOI:10.1016/j.tiv.2006.08.001 |
| [27] | Faucette S R, Sueyoshi T, Smith C M, et al. Differential regulation of hepatic CYP2B6 and CYP3A4 genes by constitutive androstane receptor but not pregnane X receptor[J]. J Pharmacol Exp Ther , 2006, 317 (3) :1200–1209. DOI:10.1124/jpet.105.098160 |
| [28] | Li H, Wang H. Activation of xenobiotic receptors:driving into the nucleus[J]. Expert Opin Drug Metab Toxicol , 2010, 6 (4) :409–426. DOI:10.1517/17425251003598886 |
| [29] | Kawamoto T, Sueyoshi T, Zelko I, et al. Phenobarbital responsive nuclear translocation of the receptor CAR in induction of the CYP2B2 gene[J]. Mol Cell Biol , 1999, 19 (9) :6318–6322. DOI:10.1128/MCB.19.9.6318 |
| [30] | Kim J, Min G, Kemper B. Chromatin assembly enhances binding to the CYP2B1 phenobarbital-responsive unit (PBRU) of nuclear factor-1, which binds simultaneously with constitutive androstane receptor (CAR)/retinoid X receptor (RXR) and enhances CAR/RXR-mediated activation of the PBRU[J]. J Biol Chem , 2001, 276 (10) :7559–7567. DOI:10.1074/jbc.M008090200 |
 2016, Vol. 47
2016, Vol. 47


