2. 四川省医学科学院(四川省人民医院), 四川 成都 610072;
3. 中国科学院昆明植物研究所 植物化学与西部植物资源持续利用国家重点实验室, 云南 昆明 650201
2. Sichuan Provincial People's Hospital, Sichuan Academy of Medical Sciences, Chengdu 610072, China;
3. State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, China
桦褐孔菌Inonotus obliquus (Fr.) Pila为生长在寒带的不育木腐菌,属于真菌门、担子菌亚门、层菌纲、非褶菌目、褐卧孔菌属的药用真菌。主要分布于北纬45°~50°的地区,包括北美(北部)、波兰、芬兰、俄罗斯(西西伯利亚、堪察加半岛、远东地区)、日本(北海道)以及中国(黑龙江、内蒙古大兴安岭、吉林省长白山)[1]。桦褐孔菌在俄罗斯作为民间用药已经几十年,具有广泛的药用价值,其在治疗多种恶性肿瘤及糖尿病、防治艾滋病、抗衰老、抑制病毒、调血脂、降血压等方面都具有明显的效果[2]。近年来关于桦褐孔菌化学成分的研究较多,其化学成分包括多种三萜类、木脂素类、黑色素类、叶酸衍生物、多糖等,其中主要为羊毛脂烷型三萜类化合物[3, 4, 5]。
三萜类成分不仅是桦褐孔菌子实体中的主要成分,更是其活性成分。其中量较高的成分桦褐孔菌醇(inotodio1)不仅具有抗肿瘤活性,还具有抗突变和抗氧化等活性[6]。为深入开发和利用桦褐孔菌子实体中的三萜类活性成分,本实验对其进行了系统的次生代谢产物研究。通过各种色谱分离技术,从桦褐孔菌子实体醇提取物中分离得到13个三萜化合物和2个甾醇化合物,分别鉴定为3-羊毛甾- 8,24-二烯-21-醛(3-oxo-lanosta-8,24-diene-21-al,1)、羊毛甾醇(lanosterol,2)、3β-羟基-羊毛甾-8,24-二烯-21-醛(3β-hydroxy-lanosta-8,24-diene-21-al,3)、白桦脂醇(betulin,4)、桦褐孔菌醇(inotodiol,5)、栓菌酸(trametenolic acid,6)、3β,21-二羟基-羊毛甾-8,24-二烯(3β,21-dihydroxy-lanosta-8,24-diene,7)、齐墩果酸(oleanic acid,8)、乌苏酸(ursolic acid,9)、白桦脂酸(betulinic acid,10)、桦褐孔菌素A(inonotusane A,11)、桦褐孔菌萜D(inoterpene D,12)、3β-乙酰氧基-11α,12α-环氧-齐墩果烷-28,13β-内酯(3-O-acetyl-11α,12α-epoxy-oleanan-28,13β- olide,13)、麦角甾醇(ergosterol,14)、麦角甾烷- 4,6,8,22-四烯-3-酮(ergosta-4,6,8,22-tetraene-3-one,15)。其中,化合物1为新化合物,命名为桦褐孔菌素D,化合物9、13、15为首次从桦褐孔菌子实体中分离得到。
1 仪器与材料VG Auto Spec 3000(英国VG公司)及Finnigan MAT 90质谱仪(德国Finnigan公司);Bruker AM-400和Avance-600核磁共振光谱仪(德国布鲁克公司)。柱色谱硅胶(200~300目)和薄层色谱硅胶GF254为青岛海洋集团有限公司产品;MCI填充材料为MCI-gel CHP-20P;SephadexLH-20凝胶为GE healthcare生产;反相RP-C18填料为Lichrospher生产。
桦褐孔菌为2012年6月采摘自内蒙古大兴安岭地区,经中国科学院昆明植物研究所何俊博士鉴定为桦褐孔菌Inonotus obliquus ( Fr. ) Pila的子实体。标本(KIB-2012-P-146)保存于昆明植物研究所植物化学与西部植物资源持续利用国家重点实验室。
2 提取与分离野生桦褐孔菌12 kg,粉碎,用25 L 95%乙醇室温提取48 h,再补加25 L 95%乙醇在80 ℃下回流提取2次,每次3 h,回收溶剂得乙醇浸膏,该浸膏用10 L 80%丙酮冷提48 h后旋转蒸干得丙酮浸膏。用适量蒸馏水分散,依次用石油醚、氯仿、正丁醇萃取(2 L×3),回收溶剂分别得到各萃取物100、225、290 g。
石油醚萃取物(100 g)经硅胶柱色谱,石油醚- 醋酸乙酯(50∶1、30∶1、15∶1、10∶1、4∶1、1∶1)梯度洗脱,并采用薄层色谱法合并相同部分,得到14个流分(Fr. A~N)。Fr. F(4.0 g)经过硅胶柱色谱,石油醚-醋酸乙酯(60∶1→1∶1)梯度洗脱,得到7个流分Fr. F1~F7。Fr. F7(400 mg)再经凝胶Sephadex LH-20色谱(流动相为氯仿-甲醇3∶2)和反相硅胶柱色谱(80%甲醇→100%甲醇)得到化合物1(36 mg)。
其他石油醚萃取物流分以及氯仿萃取物分别通过硅胶柱色谱分离,石油醚-醋酸乙酯梯度洗脱,得到的组分再经过反复硅胶柱色谱、Sephadex LH-20凝胶色谱、RP-C18反相硅胶柱色谱等手段,从石油醚部位分离纯化得到化合物2(3.12 g)、3(1.04 g)、4(2.58 g)、5(3.51 g)、6(2.21 g)、13(8.4 mg)、14(2.34 g)、15(55 mg),从氯仿部位分离纯化得到化合物7(10 mg)、8(22 mg)、9(18 mg)、10(85 mg)、11(8 mg)、12(13 mg)。
3 结构鉴定化合物1:白色粉末,15%硫酸乙醇溶液显粉色。HR-EI-MS m/z: 438.350 4 [M]+,计算值为438.349 8,结合NMR波谱数据(表 1)推测化合物1分子式为C30H46O2,不饱和度为8。在化合物1的1H-NMR (400 MHz,CDCl3) 谱图中,高场显示7个甲基单峰,中场显示1个三取代双键质子 (δH 5.34),低场显示1个醛基质子信号(δH 9.45)。化合物1的13C-NMR (100 MHz,CDCl3) 谱图显示30个碳信号,其中包括7个甲基、10个亚甲基信号、4个次甲基信号和9个季碳信号。结合1H- 和13C-NMR谱图数据推测化合物1为羊毛甾烷三萜化合物。
| 表 1 化合物1的核磁共振波谱数据 Table 1 1H-NMR and 13C-NMR spectral data for compound 1 |
与本实验中分离得到的化合物3(3β-羟基-羊毛甾-8,24-二烯-21-醛)相比,2个化合物的核磁数据非常相似,仅有的不同在于3β-羟基-羊毛甾-8,24-二烯-21-醛中的含氧次甲基碳 (C-3,δC 78.9) 在化合物1中被酮羰基碳 (δC 217.8) 取代,推测化合物1为3β-羟基-羊毛甾-8,24-二烯-21-醛的C-3位氧化成羰基。HSQC谱中将氢和碳相关信号进行归属,醛基质子信号δH 9.45 (1H,d,J = 5.6 Hz) 连接于醛基碳信号δC 206.2,双键质子信号δH 5.34 (1H,t,J = 6.0 Hz) 连接于三取代双键碳信号δC 123.4。化合物1的HMBC谱中,甲基质子H-28和H-29,以及H-2与酮羰基碳δC 217.8相关,证实化合物1的C-3位为酮羰基取代,其他HMBC相关(图 1)也进一步确认化合物1为3β-羟基-羊毛甾-8,24-二烯-21-醛的C-3位氧化成羰基,同时化合物1与3β-羟基-羊毛甾-8,24-二烯-21-醛手性碳的相关C、H数据也一致,故化合物1的结构确定为3-羊毛甾-8,24-二烯- 21-醛,为1个新化合物,命名为桦褐孔菌素D。
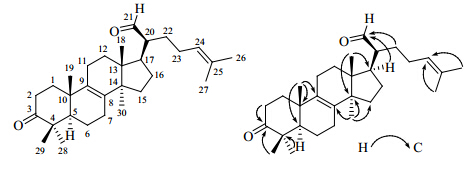 | 图 1 化合物1的结构及其关键HMBC相关Fig. 1 Structure and key HMBC correlations of compound 1 |
化合物2:白色针晶(三氯甲烷);EI-MS m/z: 426 [M]+,相对分子质量为426,分子式为C30H50O。1H-NMR (400 MHz,CDCl3) δ: 0.68 (3H,s,H-30),0.80 (3H,s,H-18),0.87 (3H,d,J = 6.5 Hz,H-21),0.90 (3H,s,H-28),0.99 (3H,s,H-19),1.03 (3H,s,H-29),1.60 (3H,s,H-27),1.68 (3H,s,H-26),3.21 (1H,dd,J = 3.4,9.3 Hz,H-3),5.08 (1H,t,J = 5.2 Hz,H-24);13C-NMR (100 MHz,CDCl3) δ: 36.3 (C-1),28.2 (C-2),79.0 (C-3),38.9 (C-4),50.9 (C-5),21.0 (C-6),27.8 (C-7),134.3 (C-8),134.3 (C-9),37.0 (C-10),18.2 (C-11),26.5 (C-12),44.4 (C-13),49.8 (C-14),30.9 (C-15),30.8 (C-16),50.3 (C-17),15.4 (C-18),18.6 (C-19),36.2 (C-20),19.1 (C-21),35.5 (C-22),25.7 (C-23),125.2 (C-24),130.9 (C-25),24.9 (C-26),17.6 (C-27),24.2 (C-28),27.9 (C-29),15.7 (C-30)。以上数据与文献报道一致[7],故鉴定化合物2为羊毛甾醇。
化合物3:白色粉末;EI-MS m/z: 440 [M]+,相对分子质量为440,分子式为C30H48O2。1H-NMR (400 MHz,CDCl3) δ: 0.67 (3H,s,H-30),0.79 (3H,s,H-18),0.89 (3H,s,H-28),0.95 (3H,s,H-19),0.99 (3H,s,H-29),1.56 (3H,s,H-27),1.67 (3H,s,H-26),3.20 (1H,dd,J = 11.5,4.5 Hz,H-3),5.04 (1H,t,J = 5.9 Hz,H-24),9.44 (1H,d,J = 5.6 Hz,H-21);13C-NMR (100 MHz,CDCl3) δ: 35.5 (C-1),29.1 (C-2),78.9 (C-3),38.8 (C-4),50.3 (C-5),18.1 (C-6),26.8 (C-7),134.7 (C-8),133.9 (C-9),37.0 (C-10),20.7 (C-11),29.6 (C-12),44.2 (C-13),49.4 (C-14),30.6 (C-15),27.8 (C-16),45.3 (C-17),16.8 (C-18),19.1 (C-19),55.5 (C-20),206.3 (C-21),35.5 (C-22),25.7 (C-23),123.5 (C-24),132.4 (C-25),25.7 (C-26),17.7 (C-27),27.9 (C-28),15.4 (C-29),24.2 (C-30)。以上数据与文献报道一致[4],故鉴定化合物3为3β-羟基-羊毛甾-8,24-二烯-21-醛。
化合物4:白色针晶(三氯甲烷);EI-MS m/z: 442 [M]+,相对分子质量为442,分子式为C30H50O2。1H-NMR (400 MHz,CDCl3) δ: 0.75 (3H,s,H-24),0.82 (3H,s,H-25),0.96 (3H,s,H-23),0.97 (3H,s,H-27),1.02 (3H,s,H-26),1.68 (3H,s,H-30),3.18 (1H,dd,J = 11.2,4.8 Hz,H-3),3.31 (1H,d,J = 10.8 Hz,H-28α),3.78 (1H,d,J = 10.8 Hz,H-28β),4.58 (1H,s,H-29α),4.68 (1H,s,H-29β);13C-NMR (100 MHz,CDCl3) δ: 38.6 (C-1),27.3 (C-2),79.0 (C-3),38.8 (C-4),55.2 (C-5),18.3 (C-6),34.2 (C-7),40.9 (C-8),50.3 (C-9),37.1 (C-10),20.8 (C-11),25.1 (C-12),37.2 (C-13),42.7 (C-14),27.0 (C-15),29.1 (C-16),47.7 (C-17),47.7 (C-18),48.7 (C-19),150.5 (C-20),29.7 (C-21),33.9 (C-22),27.9 (C-23),15.3 (C-24),16.1 (C-25),15.9 (C-26),14.7 (C-27),60.5 (C-28),109.7 (C-29),19.0 (C-30)。以上数据与文献报道一致[8],故鉴定化合物4为白桦脂醇。
化合物5:白色针晶(三氯甲烷);EI-MS m/z: 442 [M]+,相对分子质量为442,分子式为C30H50O2。1H-NMR (400 MHz,CDCl3) δ: 0.72 (3H,s,H-28),0.81 (3H,s,H-18),0.87 (3H,s,H-30),0.93 (3H,d,J = 6.7 Hz,H-21),0.98 (3H,s,H-19),0.99 (3H,s,H-29),1.65 (3H,s,H-26),1.74 (3H,s,H-27),3.24 (1H,dd,J = 11.6,4.5 Hz,H-3),3.66 (1H,m,H-22),5.17 (1H,t,J = 7.5 Hz,H-24);13C-NMR (100 MHz,CDCl3) δ: 35.5 (C-1),27.8 (C-2),78.9 (C-3),38.9 (C-4),50.3 (C-5),18.2 (C-6),27.2 (C-7),134.5 (C-8),134.1 (C-9),37.0 (C-10),21.0 (C-11),26.5 (C-12),44.8 (C-13),49.4 (C-14),30.9 (C-15),29.0 (C-16),47.2 (C-17),15.6 (C-18),18.0 (C-19),41.6 (C-20),12.6 (C-21),73.3 (C-22),26.5 (C-23),121.3 (C-24),135.2 (C-25),26.0 (C-26),19.1 (C-27),15.4 (C-28),27.9 (C-29),24.3 (C-30)。以上数据与文献报道一致[6],故鉴定化合物5为桦褐孔菌醇。
化合物6:白色针晶(三氯甲烷);EI-MS m/z: 456 [M]+,相对分子质量为456,分子式为C30H48O3。1H-NMR (400 MHz,DMSO-d6) δ: 0.66 (3H,s,H-28),0.67 (3H,s,H-18),0.80 (3H,s,H-30),0.87 (3H,s,H-19),0.88 (1H,s,H-29),1.50 (3H,s,H-26),1.61 (1H,s,H-27),2.97 (1H,m,H-3),5.05 (1H,t,J = 6.6 Hz,H-24),12.06 (1H,s,H-21);13C-NMR (100 MHz,DMSO-d6) δ: 35.2 (C-1),28.4 (C-2),76.8 (C-3),38.6 (C-4),50.1 (C-5),19.0 (C-6),28.1 (C-7),134.3 (C-8),133.4 (C-9),36.6 (C-10),20.3 (C-11),26.0 (C-12),43.8 (C-13),49.0 (C-14),32.3 (C-15),30.0 (C-16),47.5 (C-17),15.9 (C-18),17.9 (C-19),46.6 (C-20),177.1 (C-21),26.5 (C-22),25.6 (C-23),123.8 (C-24),131.2 (C-25),25.5 (C-26),17.5 (C-27),15.7 (C-28),27.6 (C-29),24.1 (C-30)。以上数据与文献报道一致[9],故鉴定化合物6为栓菌酸。
化合物7:白色粉末;EI-MS m/z: 442 [M]+,相对分子质量为442,分子式为C30H50O2。1H-NMR (400 MHz,CDCl3) δ: 0.71 (3H,s,H-28),0.81 (3H,s,H-30),0.89 (3H,s,H-29),0.98 (3H,s,H-19),1.00 (3H,s,H-18),1.61 (3H,s,H-26),1.68 (3H,s,H-27),3.23 (1H,dd,J = 11.6,4.5 Hz,H-3),3.69 (2H,m,H-21),5.11 (1H,t,J = 6.8 Hz,H-24);13C-NMR (100 MHz,CDCl3) δ: 35.5 (C-1),27.8 (C-2),78.9 (C-3),38.8 (C-4),50.3 (C-5),18.2 (C-6),26.4 (C-7),134.3 (C-8),134.2 (C-9),37.0 (C-10),20.9 (C-11),29.7 (C-12),44.2 (C-13),49.8 (C-14),30.5 (C-15),27.7 (C-16),44.3 (C-17),15.4 (C-18),19.1 (C-19),42.8 (C-20),62.5 (C-21),30.7 (C-22),25.0 (C-23),124.8 (C-24),131.4 (C-25),25.7 (C-26),17.7 (C-27),27.9 (C-28),16.0 (C-29),24.3 (C-30)。以上数据与文献报道一致[4],故鉴定化合物7为3β,21-二羟基-羊毛甾- 8,24-二烯。
化合物8:白色粉末;EI-MS m/z: 456 [M]+,相对分子质量为456,分子式为C30H48O3。1H-NMR (400 MHz,C5D5N) δ: 0.88 (3H,s,H-26),0.94 (3H,s,H-24),1.00 (3H,s,H-23),1.01 (3H,s,H-30),1.02 (3H,s,H-29),1.24 (3H,s,H-25),1.28 (3H,s,H-27),3.44 (1H,dd,J = 10.0,6.0 Hz,H-3) 5.49 (1H,t,J = 3.0 Hz,H-12);13C-NMR (100 MHz,C5D5N) δ: 39.0 (C-1),28.4 (C-2),78.1 (C-3),39.4 (C-4),55.8 (C-5),18.8 (C-6),33.3 (C-7),39.8 (C-8),48.2 (C-9),37.4 (C-10),23.7 (C-11),122.6 (C-12),144.9 (C-13),42.0 (C-14),28.1 (C-15),23.9 (C-16),46.7 (C-17),42.0 (C-18),46.5 (C-19),31.0 (C-20),34.2 (C-21),33.2 (C-22),28.8 (C-23),16.6 (C-24),15.6 (C-25),17.5 (C-26),26.2 (C-27),180.3 (C-28),33.3 (C-29),23.8 (C-30)。以上数据与文献报道一致[10],故鉴定化合物8为齐墩果酸。
化合物9:白色粉末;EI-MS m/z: 456 [M]+,相对分子质量为456,分子式为C30H48O3。1H-NMR (400 MHz,C5D5N) δ: 0.88 (3H,s,H-26),0.94 (3H,d,J = 6.5 Hz,H-29),0.99 (3H,d,J = 6.5 Hz,H-30),1.02 (3H,s,H-23),1.05 (3H,s,H-24),1.22 (3H,s,H-25),1.24 (3H,s,H-27),3.45 (1H,dd,J = 10.0,6.0 Hz,H-3),5.49 (1H,t,J = 2.5 Hz,H-12);13C-NMR (100 MHz,C5D5N) δ: 39.1 (C-1),28.2 (C-2),78.2 (C-3),39.4 (C-4),55.9 (C-5),18.8 (C-6),33.6 (C-7),40.0 (C-8),48.1 (C-9),37.3 (C-10),23.7 (C-11),125.7 (C-12),139.3 (C-13),42.5 (C-14),28.7 (C-15),24.9 (C-16),48.1 (C-17),53.6 (C-18),39.4 (C-19),39.5 (C-20),31.1 (C-21),37.5 (C-22),28.9 (C-23),16.6 (C-24),15.7 (C-25),17.5 (C-26),24.0 (C-27),180.0 (C-28),17.6 (C-29),21.5 (C-30)。以上数据与文献报道一致[11],故鉴定化合物9为乌苏酸。
化合物10:白色粉末;EI-MS m/z: 456 [M]+,相对分子质量为456,分子式为C30H48O3。1H-NMR (400 MHz,C5D5N) δ: 0.81 (3H,s,H-23),1.00 (3H,s,H-24),1.05 (3H,s,H-25),1.06 (3H,s,H-26),1.22 (3H,s,H-27),1.78 (3H,s,H-30),3.45 (1H,t,J = 8.0 Hz,H-3),4.76 (1H,s,H-29β),4.94 (1H,s,H-29α);13C-NMR (100 MHz,C5D5N) δ: 39.3 (C-1),28.3 (C-2),78.1 (C-3),39.6 (C-4),55.9 (C-5),18.8 (C-6),34.8 (C-7),41.1 (C-8),51.0 (C-9),37.5 (C-10),21.2 (C-11),26.1 (C-12),38.6 (C-13),42.9 (C-14),31.2 (C-15),32.9 (C-16),56.7 (C-17),47.8 (C-18),49.8 (C-19),151.4 (C-20),30.3 (C-21),37.6 (C-22),28.7 (C-23),16.4 (C-24),16.4 (C-25),16.5 (C-26),14.9 (C-27),178.9 (C-28),110.0 (C-29),19.5 (C-30)。以上数据与文献报道一致[8],故鉴定化合物10为白桦脂酸。
化合物11:白色无定形粉末;HR-EI-MS m/z: 458.376 0,分子式为C30H50O3。1H-NMR (400 MHz,CDCl3) δ: 0.70 (3H,s,H-18),0.81 (3H,s,H-29),0.91 (3H,s,H-30),0.98 (3H,s,H-19),1.00 (3H,s,H-28),1.14 (3H,s,H-27),1.41 (3H,s,H-26),3.24 (1H,dd,J = 11.5,4.4 Hz,H-3),4.38 (1H,t,J = 3.0 Hz,H-21);13C-NMR (100 MHz,CDCl3) δ: 35.7 (C-1),27.9 (C-2),79.1 (C-3),39.0 (C-4),50.5 (C-5),18.3 (C-6),26.6 (C-7),134.3 (C-8),134.6 (C-9),37.2 (C-10),21.1 (C-11),30.5 (C-12),44.3 (C-13),49.5 (C-14),31.2 (C-15),27.4 (C-16),44.2 (C-17),15.6 (C-18),19.3 (C-19),48.8 (C-20),75.8 (C-21),21.2 (C-22),28.4 (C-23),53.5 (C-24),72.1 (C-25),29.4 (C-26),30.1 (C-27),28.1 (C-28),17.0 (C-29),24.6 (C-30)。以上数据与文献报道一致[12],故鉴定化合物11为桦褐孔菌素A。
化合物12:白色粉末;ESI-MS m/z: 481 [M+Na]+,相对分子质量为458,分子式为C30H50O3。1H-NMR (400 MHz,CDCl3) δ: 0.77 (3H,s,H-18),0.80 (3H,s,H-29),0.85 (3H,s,H-30),0.97 (3H,s,H-19),0.99 (3H,s,H-28),1.67 (3H,s,H-26),1.75 (3H,s,H-27),3.22 (1H,dd,J = 11.5,4.4 Hz,H-3),3.71 (1H,t,J = 10.5 Hz,H-21α),3.82 (1H,d,J = 10.4 Hz,H-22),4.01 (1H,m,H-21β),5.27 (1H,m,H-24);13C-NMR (100 MHz,CDCl3) δ: 35.7 (C-1),28.0 (C-2),79.1 (C-3),39.0 (C-4),50.5 (C-5),18.4 (C-6),27.5 (C-7),134.2 (C-8),134.8 (C-9),37.2 (C-10),21.1 (C-11),26.6 (C-12),44.8 (C-13),49.7 (C-14),30.9 (C-15),30.9 (C-16),47.4 (C-17),16.0 (C-18),19.3 (C-19),43.2 (C-20),63.6 (C-21),75.5 (C-22),30.0 (C-23),121.1 (C-24),135.8 (C-25),18.2 (C-26),26.1 (C-27),28.1 (C-28),15.6 (C-29),24.4 (C-30)。以上数据与文献报道一致[13],故鉴定化合物12为桦褐孔菌萜D。
化合物13:白色晶体(三氯甲烷);ESI-MS m/z: 535 [M+Na]+,相对分子质量为512,分子式为C32H48O5。1H-NMR (600 MHz,CDCl3) δ: 0.80 (3H,s,H-23),0.81 (3H,s,H-24),0.85 (3H,s,H-30),0.93 (3H,s,H-29),0.98 (3H,s,H-25),1.00 (3H,s,H-26),1.03 (3H,s,H-27),1.85 (1H,dt,J = 13.2,3.3 Hz,H-16α),1.99 (3H,s,3-OAc),2.06 (1H,td,J = 13.2,5.7 Hz,H-16β),2.25 (1H,dd,J = 13.7,2.9 Hz,H-18),2.95 (1H,dd,J = 4.0,1.5 Hz,H-11),4.46 (1H,dd,J = 11.3,5.1 Hz,H-3);13C-NMR (150 MHz,CDCl3) δ: 38.0 (C-1),23.3 (C-2),80.6 (C-3),38.0 (C-4),54.8 (C-5),17.6 (C-6),31.2 (C-7),41.5 (C-8),50.7 (C-9),36.5 (C-10),52.8 (C-11),57.2 (C-12),87.7 (C-13),40.7 (C-14),27.1 (C-15),21.4 (C-16),44.0 (C-17),49.7 (C-18),37.9 (C-19),31.6 (C-20),34.4 (C-21),26.8 (C-22),27.9 (C-23),16.4 (C-24),17.4 (C-25),20.2 (C-26),19.0 (C-27),179.5 (C-28),33.3 (C-29),23.7 (C-30),21.4 (C-31),171.1 (C-32)。以上数据与文献报道一致[14],故鉴定化合物13为3β-乙酰氧基- 11α,12α-环氧-齐墩果烷-28,13β-内酯。
化合物14:白色针晶(三氯甲烷);EI-MS m/z: 396 [M]+,相对分子质量为396,分子式为C28H44O。1H-NMR (400 MHz,C5D5N) δ: 0.67 (3H,s,H-18),0.86 (3H,d,J = 6.0 Hz,H-27),0.87 (3H,d,J = 6.0 Hz,H-26),0.96 (3H,d,J = 6.8 Hz,H-28),1.03 (3H,s,H-19),1.07 (3H,d,J = 6.8 Hz,H-21),3.95 (1H,m,H-3),5.19 (1H,m,H-22),5.25 (1H,m,H-23),5.50 (1H,dd,J = 5.2,2.4 Hz,H-7),5.70 (1H,dd,J = 5.2,2.4 Hz,H-6);13C-NMR (100 MHz,C5D5N) δ: 39.0 (C-1),33.0 (C-2),69.9 (C-3),42.0 (C-4),140.9 (C-5),119.7 (C-6),117.2 (C-7),141.3 (C-8),46.6 (C-9),37.5 (C-10),21.8 (C-11),39.3 (C-12),43.0 (C-13),54.8 (C-14),23.4 (C-15),28.8 (C-16),55.8 (C-17),12.2 (C-18),16.6 (C-19),40.9 (C-20),21.4 (C-21),136.2 (C-22),132.1 (C-23),43.1 (C-24),33.4 (C-25),19.9 (C-26),20.2 (C-27),17.9 (C-28)。以上数据与文献报道一致[15],故鉴定化合物14为麦角甾醇。
化合物15:黄色固体;ESI-MS m/z: 393 [M+H]+,相对分子质量为392,分子式为C28H40O。1H-NMR (400 MHz,CDCl3) δ: 0.76 (3H,d,J = 6.7 Hz,H-26),0.78 (3H,d,J = 6.7 Hz,H-27),0.86 (3H,d,J = 6.8 Hz,H-28),0.89 (3H,s,H-18),0.93 (3H,s,H-19),0.99 (3H,d,J = 6.7 Hz,H-21),5.27 (1H,dd,J = 5.9,14.2 Hz,H-24),5.29 (1H,dd,J = 5.9,14.2 Hz,H-23),5.67 (1H,s,H-4),5.96 (1H,d,J = 9.5 Hz,H-6),6.54 (1H,d,J = 9.5 Hz,H-7);13C-NMR (100 MHz,CDCl3) δ: 34.3 (C-1),34.4 (C-2),199.7 (C-3),123.1 (C-4),164.6 (C-5),124.6 (C-6),134.2 (C-7),156.3 (C-8),124.6 (C-9),36.9 (C-10),23.1 (C-11),35.7 (C-12),44.1 (C-13),44.5 (C-14),25.5 (C-15),27.9 (C-16),55.8 (C-17),16.8 (C-18),19.1 (C-19),39.4 (C-20),19.8 (C-21),135.1 (C-22),132.7 (C-23),43.0 (C-24),33.2 (C-25),21.4 (C-26),20.1 (C-27),17.8 (C-28)。以上数据与文献报道一致[16],故鉴定化合物15为麦角甾烷-4,6,8,22-四烯-3-酮。
| [1] | 赵芬琴, 朴惠善. 桦褐孔菌的研究进展 [J]. 中国中医药信息杂志, 2005, 12(2): 96-98. |
| [2] | 黄年来. 俄罗斯神秘的民间药用真菌——桦褐孔菌 [J]. 中国食用菌, 2002, 21(4): 7-8. |
| [3] | 赵芬琴, 朴惠善. 桦褐孔菌的化学成分研究 [J]. 时珍国医国药, 2006, 17(7): 1178-1181. |
| [4] | 何 坚, 冯孝章. 桦褐孔菌化学成分的研究 [J]. 中草药, 2001, 32(1): 4-6. |
| [5] | 刘迎秋, 包海鹰. 桦褐孔菌Inonotus obliquus化学成分及药理作用 [J]. 中国食用菌, 2008, 27(4): 34-39. |
| [6] | 赵芬琴, 邓丽颖, 杨灿宇, 等. 桦褐孔菌中的活性化合物桦褐孔菌醇 [J]. 药学服务与研究, 2009, 9(6): 455-458. |
| [7] | 杨秀伟, 韩美华, 靳彦平. 金线莲化学成分的研究 [J]. 中药材, 2007, 30(7): 797-800. |
| [8] | Salimuzzaman S, Farrukh H, Sabira B, et al. Oleanderol, a new pentacyclic triterpene from the leaves of Nerium oleander [J]. J Nat Prod, 1988, 51(2): 229-233. |
| [9] | 张 旭, 赵芬琴, 韩 光, 等. 桦褐孔菌的化学成分及抗炎活性 [J]. 天然产物研究与开发, 2010, 22(3): 433-436. |
| [10] | 白玉华, 于 辉, 常乃丹, 等. 日本苦苣菜的化学成分 [J]. 中国药科大学学报, 2008, 39(3): 279-281. |
| [11] | 张小坡, 裴月湖, 刘明生, 等. 海芒果叶中三萜类成分的研究 [J]. 天然产物研究与开发, 2011, 23(3): 443-445. |
| [12] | Zhao F Q, Mai Q Q, Ma J H, et al. Triterpenoids from Inonotus obliquus and their antitumor activities [J]. Fitoterapia, 2015, 101: 34-40. |
| [13] | Nakamura S, Iwami J, Matsuda H, et al. Absolute stereostructures of inoterpenes A-F from sclerotia of Inonotus obliquus [J]. Tetrahedron, 2009, 65(12): 2443-2450. |
| [14] | Narváez-Mastache J M, Soto C, Delgado G. Antioxidant evaluation of Eysenhardtia species (Fabaceae): relay synthesis of 3-O-acetyl-11alpha, 12alpha-epoxy-oleanan-28,13β-olide isolated from E. platycarpa and its protective effect in experimental diabetes [J]. Biol Pharm Bull, 2007, 30(8): 1503-1510. |
| [15] | 徐 菁, 高鸿悦, 马淑丽, 等. 马兰化学成分及生物活性研究 [J]. 中草药, 2014, 45(22): 3246-3250. |
| [16] | Graziose R, Rojas-Silva P, Rathinasabapathy T, et al. Antiparasitic compounds from Cornus florida L. with activities against Plasmodium falciparum and Leishmania tarentolae [J]. J Ethnopharmacol, 2012, 142(2): 456-461. |
 2015, Vol. 46
2015, Vol. 46


