2. 中山中智药业集团有限公司, 广东 中山 528437;
3. 华南理工大学分析测试中心, 广东 广州 510640
2. Zhongzhi Pharmaceutical Company Group, Zhongshan 528437, China;
3. Analysis and Test Center of South China University of Technology, Guangzhou 510640, China
蒲桃(原变种)Syzygium jambos var. jambos (L.) Alston为桃金娘科蒲桃属植物,在我国主要分布于台湾、福建、广东、广西、贵州和云南等省区,蒲桃茎作为一种传统民间药材在岭南地区使用已有悠久历史,具有温中散寒、降逆止呕、温肺止咳,可用于治疗胃寒呃逆、肺虚寒咳等病症,并收载于广东省地方中药材标准[1]。现代药理学研究表明蒲桃茎提取物具有降血糖、抗氧化、抗菌以及抗炎等作用[2,3,4,5]。综合查阅国内外文献发现蒲桃茎的药效物质基础研究鲜有报道,仅有关于其挥发油成分的研究报道[6,7]。为了阐明该药材的药效物质基础,本实验对蒲桃茎进行研究,从其醋酸乙酯和甲醇提取物中分离得到12个化合物,分别鉴定为麦珠子酸(alphitolic acid,1)、urolithin A(2)、邻苯二甲酸二正丁酯(dibutyl phthalate,3)、桦木酸(betulinic acid,4)、熊果酸(ursolic acid,5)、1-(4-methoxy- phenyl)-1,2-propanediol(6)、2,6-dimethoxy-1,4- benzoquinone(7)、邻苯二甲酸二异丁酯(diisobutyl phthalate,8)、β-谷甾醇(β-sitosterol,9)、3-乙酰-熊果酸(3-acetyl-ursolic acid,10)、积雪草酸(asiatic acid,11)和阿江榄仁酸(arjunolic acid,12),其中,除化合物4,其他11个化合物均为首次从该植物分离获得,三萜酸类化合物是蒲桃茎的主要化学成分,体外细胞活性测试结果表明在50 μmol/L浓度时化合物1、7、10、11和12对Huh-7细胞均未表现出明显细胞毒作用
1 仪器与药材Bruker Avance 400和600型核磁共振波谱仪(瑞士Bruker公司);电子轰击电离质谱仪(美国Thermofisher公司);LH—20型葡聚糖凝胶(GE Healthcare公司);D101型大孔吸附树脂(天津南大树脂科技有限公司);制备液相(大连依利特公司);实验所用试剂均为分析纯或色谱纯。3111型CO2细胞培养箱,美国Thermo Scientific公司产品;CKX41型相差倒置显微镜,日本Olympus公司产品;TC10型细胞计数仪、Imark酶标仪,美国Bio-Rad公司产品;TGL—16G型高速台式离心机,上海安亭科学仪器公司产品。DMEM培养液(C11995)、PBS缓冲液(C10010)、胎牛血清(10099)、0.25% Trypsin-EDTA(25200)、Pen Strep(15140)均为美国Gibco公司产品;噻唑蓝(MTT),美国Sigma公司产品。
蒲桃茎由中山市中智药业集团有限公司赖卓宏于2011年4月采集于广东省郁南县,经中国科学院华南植物研究所叶华谷研究员鉴定为桃金娘科蒲桃属蒲桃(原变种)Syzygium jambos var. jambos (L.) Alston的茎,树龄20年左右。药材标本(0120110402)存放于中山市中智药业集团技术中心实验室。
2 提取与分离取4.4 kg蒲桃茎干燥粗粉,依次用醋酸乙酯(7.5 L×4)和甲醇(7.5 L×4)超声提取(40 kHz,45 ℃,30 min),超声萃取液用布氏漏斗抽滤,滤液用旋转蒸发仪浓缩成干浸膏,称定质量,分别得到醋酸乙酯提取物28.7 g和甲醇提取物264.4 g。
取醋酸乙酯提取物26.0 g,采用硅胶柱色谱(石油醚-醋酸乙酯100∶0→0∶100)进行梯度洗脱,共获得25个组分Fr. 1~25。Fr. 5(0.32 g)进一步通过硅胶柱色谱法(石油醚-醋酸乙酯100∶0→0∶100)分离获得化合物3(42.0 mg); Fr. 8(0.39 g)经过硅胶柱色谱法(石油醚-醋酸乙酯100∶0→0∶100)和重结晶法分离纯化后获得化合物4(5.1 mg)和5(4.6 mg);Fr. 10(0.87 g)经过氧化铝柱色谱法(石油醚-醋酸乙酯100∶0→0∶100,醋酸乙酯-甲醇80∶20洗脱),得到微组分Fr. 10-1~10-18,其中微组分Fr. 10-18(66.7 mg)再利用制备薄层色谱法(石油醚-醋酸乙酯3∶1)纯化得到化合物10(7.7 mg);Fr. 11(0.94 g)利用氯仿重结晶,获得化合物9(76.4 mg);Fr. 21(0.64 g)先后利用硅胶柱色谱(石油醚-醋酸乙酯100∶0 →0∶100)和葡聚糖凝胶柱色谱(醋酸乙酯-甲醇3∶1)分离纯化后得到化合物1(5 mg)、2(1.8 mg)、7(4.2 mg)和8(4.5 mg);Fr. 23(0.74 g)先后采用葡聚糖凝胶柱色谱(醋酸乙酯-甲醇3∶1)和制备液相色谱(体积流量为3 mL/min,波长为279 nm,流动相为甲醇-水,梯度洗脱,0~15 min,30%~63%甲醇;15~20 min,63%~100%甲醇;20~30 min,100%甲醇;30~32 min,100%~30%甲醇;32~35 min,30%甲醇)得到化合物6(3.3 mg);取15 g甲醇粗提物,加适量纯水混悬,正丁醇(3×100 mL)进行萃取,合并浓缩,得到正丁醇萃取物1.1 g,然后采用大孔树脂柱色谱法(乙醇-水0∶100→100∶0)进行洗脱,分离得到4个组分Fr. 1~4),其中Fr. 3(75%乙醇洗脱部分,187.5 mg)采用硅胶柱色谱(石油醚-醋酸乙酯100∶0→0∶100、醋酸乙酯-甲醇50∶50→0∶100)分离,得化合物11和12的混合物(11.1 mg)。
3 结构鉴定化合物1:白色粉末;Liebermann-Burchard反应阳性;EI-MS m/z: 472 [M]+。1H-NMR (600 MHz,CD3OD) δ: 4.71 (1H,d,J = 1.4 Hz,H-29a),4.59 (1H,d,J = 1.4 Hz,H-29b),3.90 (1H,m,H-2),3.30 (1H,m,H-3),3.01 (1H,m,H-19),1.69 (3H,s,H-30),1.02 (3H,s,H-27),0.96 (3H,s,H-23),0.95 (3H,s,H-26),0.89 (3H,s,H-25),0.83 (3H,s,H-24);13C-NMR (150 MHz,CD3OD) δ: 42.8 (C-1),67.3 (C-2),80.1 (C-3),39.5 (C-4),49.4 (C-5),19.1 (C-6),35.4 (C-7),43.7 (C-8),51.6 (C-9),39.6 (C-10),22.1 (C-11),26.8 (C-12),39.6 (C-13),42.1 (C-14),30.8 (C-15),33.3 (C-16),57.5 (C-17),50.5 (C-18),48.5 (C-19),152.0 (C-20),31.7 (C-21),38.1 (C-22),29.2 (C-23),22.2 (C-24),17.7 (C-25),16.7 (C-26),15.1 (C-27),180.1 (C-28),110.2 (C-29),19.6 (C-30)。以上数据与文献报道的一致[8],故鉴定化合物1为麦珠子酸。
化合物2:黄色粉末;EI-MS m/z: 228 [M]+。1H-NMR (600 MHz,DMSO-d6) δ: 6.72 (1H,d,J = 2.3 Hz,H-9),6.80 (1H,dd,J = 8.8,2.3 Hz,H-7),7.32 (1H,dd,J = 8.8,2.6 Hz,H-4),7.50 (1H,d,J = 2.6 Hz,H-2),8.02 (1H,d,J = 8.8 Hz,H-6),8.10 (1H,d,J = 8.8 Hz,H-5);13C-NMR (150 MHz,DMSO-d6) δ: 160.7 (C-1),113.6 (C-2),157.0 (C-3),124.2 (C-4),123.6 (C-5),123.9 (C-6),113.1 (C-7),158.6 (C-8),102.9 (C-9),120.2 (C-10),127.0 (C-11),109.9 (C-12),151.0 (C-13)。以上数据与文献报道一致[9],故鉴定化合物2为urolithin A。
化合物3:红褐色粉末;EI-MS m/z: 278 [M]+。1H-NMR (400 MHz,CDCl3) δ: 7.71 (2H,s,H-3,6),7.53 (2H,s,H-4,5),4.31 (4H,m,H-1′,1′′),1.73 (4H,m,H-2′,2′′),1.44 (4H,m,H-3′,3′′),0.97 (4H,m,H-4′,4′′);13C-NMR (100 MHz,CDCl3) δ: 167.7 (C-1,8),132.4 (C-2,7),130.9 (C-4,5),128.8 (C-3,6),65.6 (C-1′,1′′),30.6 (C-2′,2′′),19.2 (C-3′,3′′),13.7 (C-4′,4′′)。以上数据与文献报道一致[10],故鉴定化合物3为邻苯二甲酸二正丁酯。
化合物4:无色晶体(醋酸乙酯);Liebermann- Burchard反应阳性;EI-MS m/z: 456 [M]+。1H-NMR (400 MHz,DMSO-d6) δ: 4.68 (1H,s,H-29b),4.56 (1H,s,H-29a),δ 4.27 (1H,d,J = 5.1 Hz,-OH),3.30 (1H,m,H-3),2.97 (1H,m,H-19),1.64 (3H,s,H-30),0.93 (3H,s,H-27),0.87 (6H,s,H-23,26),0.76 (3H,s,H-25),0.65 (3H,s,H-24);13C-NMR (100 MHz,DMSO-d6) δ: 37.6 (C-1),27.2 (C-2),76.8 (C-3),38.5 (C-4),54.9 (C-5),18.0 (C-6),33.9 (C-7),40.3 (C-8),49.9 (C-9),36.3 (C-10),20.5 (C-11),25.1 (C-12),38.3 (C-13),42.0 (C-14),30.1 (C-15),31.7 (C-16),55.4 (C-17),46.6 (C-18),48.6 (C-19),150.3 (C-20),29.2 (C-21),36.7 (C-22),28.1 (C-23),15.7 (C-24),15.8 (C-25),15.9 (C-26),14.4 (C-27),177.2 (C-28),109.6 (C-29),19.0 (C-30)。以上数据与文献报道一致[11],故鉴定化合物4为桦木酸。
化合物5:白色粉末;Liebermann-Burchard反应阳性;EI-MS m/z: 456 [M]+。1H-NMR (400 MHz,DMSO-d6) δ: 5.12 (1H,t,J = 3.3 Hz,H-12),4.30 (1H,d,J = 5.0 Hz,-OH),2.99 (1H,m,H-3),1.03 (3H,s,H-27),0.90 (3H,d,J = 7.6 Hz,H-30),0.89 (3H,s,H-25),0.86 (3H,s,H-26),0.80 (3H,d,J = 6.4 Hz,H-29),0.74 (3H,s,H-23),0.67 (3H,s,H-24);13C-NMR (100 MHz,DMSO-d6) δ: 38.4 (C-1),27.5 (C-2),76.9 (C-3),38.5 (C-4),54.8 (C-5),18.0 (C-6),32.7 (C-7),41.7 (C-8),46.8 (C-9),36.3 (C-10),22.9 (C-11),124.6 (C-12),138.2 (C-13),40.8 (C-14),28.3 (C-15),23.8 (C-16),47.0 (C-17),52.4 (C-18),38.4 (C-19),38.2 (C-20),30.2 (C-21),36.5 (C-22),27.0 (C-23),15.2 (C-24),16.1 (C-25),16.9 (C-26),23.3 (C-27),178.3 (C-28),17.0 (C-29),21.1 (C-30)。以上数据与文献报道一致[12],故鉴定化合物5为熊果酸。
化合物6:黄绿色粉末;EI-MS m/z: 182 [M]+。1H-NMR (400 MHz,CD3OD) δ: 0.96 (3H,d,J = 6.3 Hz,H-3),3.78 (1H,m,H-2),4.30 (1H,d,J = 7.2 Hz,H-1),6.65 (2H,d,J = 8.3 Hz,H-3′,5′),7.02 (2H,d,J = 8.3 Hz,H-2′,6′);13C-NMR (100 MHz,CD3OD) δ: 78.6 (C-1),71.6 (C-2),17.8 (C-3),133.9 (C-1′),127.9 (C-2′,6′),113.2 (C-3′,5′),159.3 (C-4′),54.3 (-OCH3)。以上数据与文献报道一致[13],故鉴定化合物6为1-(4-methoxyphenyl)-1,2-propanediol。
化合物7:黄色针晶(氯仿);EI-MS m/z: 168 [M]+。1H-NMR (400 MHz,CDCl3) δ: 3.82 (6H,s -OCH3),5.86 (2H,s,H-3,5);13C-NMR (100 MHz,CDCl3)δ: 176.6 (C-1),157.3 (C-2,6),107.4 (C-3,5),186.8 (C-4),56.4 (2×-OCH3)。以上数据与文献报道一致[14],故鉴定化合物7为2,6-二甲氧基-1,4-苯醌。
化合物8:白色粉末;1H-NMR (400 MHz,CD3COCD3)δ: 7.75 (2H,m,H-3,6),7.65 (2H,m,H-4,5),4.31 (4H,d,J = 7.6 Hz,H-1′,1′′),1.73 (4H,m,H-2′,2′′),0.85 (12H,d,J = 7.6 Hz,H-3′,3′′,4′,4′′);13C-NMR (100 MHz,CD3COCD3) δ: 167.1 (C-1,8),132.5 (C-2,7),131.1 (C-4,5),128.7 (C-3,6),71.2 (C-1′,1′′),27.6 (C-2′,2′′),13.5 (C-3′,3′′,4′,4′′)。以上数据与文献报道一致[10],故鉴定化合物8为邻苯二甲酸二异丁酯。
化合物9:白色针晶(氯仿);EI-MS m/z: 414 [M]+。1H-NMR (400 MHz,CDCl3) δ: 3.51 (1H,m,H-3,),5.35 (1H,t,J = 2.6 Hz,H-6),1.01 (3H,s,H-18),0.68 (3H,s,H-19),0.84 (3H,d,J = 7.5 Hz,H-21),0.81 (3H,d,J = 6.5 Hz,H-26),0.92 (3H,d,J = 6.5 Hz,H-27),0.85 (3H,t,J = 7.6 Hz,H-29);13C-NMR (100 MHz,CDCl3) δ: 37.3 (C-1),31.7 (C-2),71.8 (C-3),42.3 (C-4),140.8 (C-5),121.7 (C-6),31.9 (C-7),31.9 (C-8),50.2 (C-9),36.5 (C-10),21.1 (C-11),39.8 (C-12),42.3 (C-13),56.8 (C-14),24.3 (C-15),28.2 (C-16),56.1 (C-17),19.4 (C-18),12.0 (C-19),36.1 (C-20),18.8 (C-21),34.0 (C-22),26.2 (C-23),45.9 (C-24),23.1 (C-25),19.8 (C-26),19.0 (C-27),29.2 (C-28),11.8 (C-29)。以上数据与文献报道一致[15],故鉴定化合物9为β-谷甾醇。
化合物10:白色粉末;Liebermann-Burchard反应阳性;EI-MS m/z: 498 [M]+。1H-NMR (400 MHz,CDCl3) δ: 5.17 (1H,t,J = 3.3 Hz,H-12),4.43 (1H,t,J = 7.2 Hz,H-3),1.02 (3H,s,H-27),0.91 (3H,d,J = 7.6 Hz,H-30),0.89 (3H,s,H-25),0.85 (3H,s,H-26),0.82 (3H,d,J = 6.4 Hz,H-29),0.74 (3H,s,H-23),0.67 (3H,s,H-24),1.97 (3H,s,-COCH3);13C-NMR (100 MHz,DMSO-d6)δ: 38.4 (C-1),27.5 (C-2),80.2 (C-3),38.0 (C-4),55.8 (C-5),18.0 (C-6),32.7 (C-7),40.7 (C-8),46.8 (C-9),37.0 (C-10),22.9 (C-11),125.9 (C-12),138.1 (C-13),42.6 (C-14),28.8 (C-15),24.8 (C-16),47.2 (C-17),52.4 (C-18),38.4 (C-19),38.2 (C-20),30.2 (C-21),36.7 (C-22),28.0 (C-23),17.2 (C-24),16.1 (C-25),16.9 (C-26),23.1 (C-27),182.9 (C-28),17.1 (C-29),21.1 (C-30),171.2 (-COCH3),21.3 (-COCH3)。以上数据与文献报道一致[16],故鉴定化合物10为3-乙酰-熊果酸。
化合物11和12:白色粉末;Liebermann- Burchard反应阳性;EI-MS m/z: 488 [M]+;通过解析其1H-、13C-NMR,HSQC和HMBC等波谱学数据,可判断为积雪草酸(11)和阿江榄仁酸(12)2个化合物的混合物,其NMR数据分别与文献报道积雪草酸和阿江榄仁酸的数据基本一致[17,18];化合物11:13C-NMR (125 MHz,CD3OD) δ: 47.6 (C-1),68.2 (C-2),76.7 (C-3),42.7 (C-4),46.6 (C-5),17.7 (C-6),32.4 (C-7),39.4 (C-8),46.2 (C-9),36.2 (C-l0),23.9 (C-11),125.1 (C-12),138.1 (C-13),42.7 (C-14),27.8 (C-15),25.1 (C-16),48.1 (C-17),52.9 (C-18),39.0 (C-19),38.9 (C-20),30.4 (C-21),37.6 (C-22),64.8 (C-23),12.6 (C-24),16.7 (C-25),16.6 (C-26),23.3 (C-27),180.5 (C-28),23.2 (C-29),22.6 (C-30)。化合物12:13C-NMR (125 MHz,CD3OD) δ: 47.4 (C-1),68.2 (C-2),76.7 (C-3),42.7 (C-4),46.5 (C-5),17.6 (C-6),32.2 (C-7),39.0 (C-8),46.2 (C-9),36.2 (C-10),23.9 (C-11),122.0 (C-12),143.9 (C-13),42.7 (C-14),27.4 (C-15),25.1 (C-16),48.1 (C-17),52.9 (C-18),46.7 (C-19),30.1 (C-20),30.4 (C-21),37.4 (C-22),64.8 (C-23),12.5 (C-24),17.6 (C-25),16.6 (C-26),23.0 (C-27),180.3 (C-28),33.5 (C-29),23.9 (C-30)。另外,综合化合物的核磁数据,可推断化合物11和12的C-12位相连的烯氢信号分别为δ 5.24 (1H,t,J = 3.6 Hz,H-12) 和5.28 (1H,t,J = 3.6 Hz,H-12),其积分比为1.4∶2.0,故推断化合物11和12的量之比约为0.7∶1。
4 体外细胞毒活性测试[19]选择蒲桃茎代表性化合物1、7、10及11+12混合物进行活性测试。体外培养人肝癌Huh-7细胞株,细胞生长至约80%密度时收集对数生长期的Huh-7细胞,消化后计数,在96孔板中每孔加入约5×103个细胞,放入细胞培养箱培养。细胞接种于96孔板24 h后,吸弃各孔中的培养液,空白对照组每孔加入200 μL含0.5% DMSO和10% FBS的DMEM培养液,给药组每孔加入200 μL含10% FBS和不同浓度(0、1、5、10、20、30、40、50 μmol/L)试药的DMEM培养液,每组平行设置3个孔,37 ℃、5% CO2培养24 h,每孔加入20 μL 5 g/L MTT,培养4 h,吸弃培养基,每孔加入150 μL DMSO,振荡10 min。酶标仪490 nm测定吸光度(A)值。
MTT测试结果(图 1)显示所有测试化合物在50 μmol/L时作用24 h后,均对人肝脏肿瘤Huh-7 细胞没有显著细胞毒活性。有文献报道三萜酸类化合物如熊果酸对Huh-7细胞没有明显细胞毒活性(IC50=75 μmol/L)[20],与本实验结果一致。
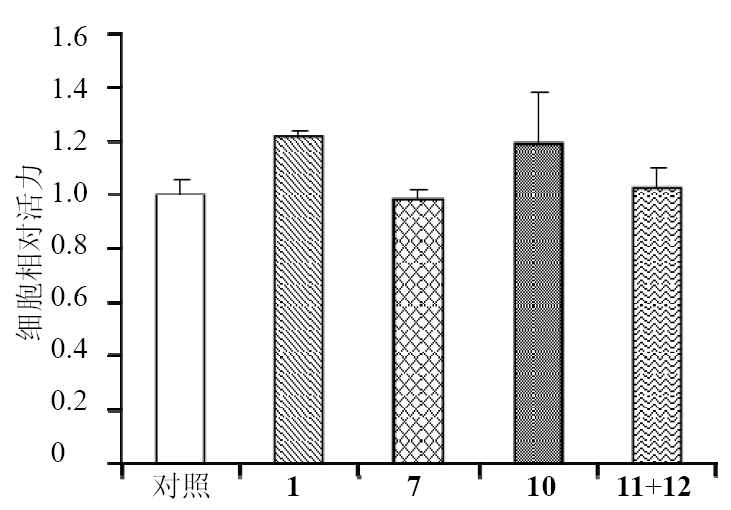
| 图 1 MTT法评价分离化合物的体外细胞毒活性 Fig. 1 In vitro cytotoxic activities of isolated compounds evaluated by MTT assay |
| [1] | 广东省中药材标准 [S]. 2004. |
| [2] | 邓家刚, 李学坚, 覃振林. 蒲桃仁提取物降血糖作用的实验研究 [J]. 广西植物, 2006, 26(2): 214-216. |
| [3] | Jayasinghe L, Ratnayake R M, Medawala M M, et al. Dihydrochalcones with radical scavenging properties from the leaves of Syzygium jambos [J]. Nat Prod Res, 2007, 21(6): 551-554. |
| [4] | Kuiate J R, Mouokeu S, Wabo H K, et al. Antidermatophytic triterpenoids from Syzygium jambos (L.) Alston (Myrtaceae) [J]. Phytother Res, 2007, 21(2): 149-152. |
| [5] | Avila-Pena D, Pena N, Quintero L, et al. Antinociceptive activity of Syzygium jambos leaves extract on rats [J]. J Ethnopharmacol, 2007, 112(2): 380-385. |
| [6] | 林大都, 刘嘉炜, 李武国, 等. 蒲桃茎超临界CO2萃取物的GC-MS分析 [J]. 中国药房, 2013, 31(24): 2946-2948. |
| [7] | 刘艳清. 蒲桃茎、叶和花挥发油化学成分的气相色谱-质谱分析 [J]. 精细化工, 2008, 25(3): 243-246. |
| [8] | Suksamrarn S, Panseeta P, Kunchanawa S, et al. Ceanothane- and lupane-type triterpenes with antiplasmodial and antimycobacterial activities from Ziziphus cambodiana [J]. Chem Pharm Bull, 2006, 54(4): 535-537. |
| [9] | Pandey J, Jha A K, Hajela K. Synthesis and biological activities of some new dibenzopyranones and dibenzopyrans: search for potential oestrogen receptor agonists and antagonists [J]. Bioorg Med Chem, 2004, 12: 2239-2249. |
| [10] | Shi D Y, Han L J, Sun J, et al. Chemical constituents from marine alga Chaetomorpha basiretorsa [J]. China J Chin Mater Med, 2005, 30(5): 347-350. |
| [11] | 胡金峰, 叶仲林, 沈凤嘉. 云南甘草中新三萜成分的研究 [J]. 药学学报, 1995, 30(1): 27-33. |
| [12] | 杨淑敏, 刘锡葵, 卿 晨, 等. 水杨柳根的化学成分 [J]. 药学学报, 2007, 42(3): 292-296. |
| [13] | Sy L K, Brown G D. Novel phenylpropanoids and lignans from Illicium verum [J]. J Nat Prod, 1998, 61(8): 987-992. |
| [14] | Harasawa A, Tagashira A. Isolation of 2, 6-dimethoxy-1, 4-benzoquinone from Hydrangea (Hydrangea macrophylla Seringe var. otaksa Makino) and its deodorant activity against methylmercaptan [J]. Biosci Biotech Biochem, 1994, 58: 2073-2074. |
| [15] | 刘嘉炜, 彭丽华, 冼美婷, 等. 露兜簕根化学成分研究 [J]. 中草药, 2012, 43(4): 636-639. |
| [16] | Ahmed Z, Ali D, Malik A. Structure determination of ursene-type triterpenes by NMR techniques [J]. Magn Reson Chem, 2006, 44(7): 717-719. |
| [17] | Rumalla C S, Ali Z, Weerasooriya A D, et al. Two new triterpene glycosides from Centella asiatica [J]. Planta Med, 2010, 76(10): 1018-1021. |
| [18] | Ramesh A S, Christopher J G, Radhika R, et al. Isolation, characterisation and cytotoxicity study of arjunolic acid from Terminalia arjuna [J]. Nat Prod Res, 2012, 26(16): 1549-1552. |
| [19] | Sylvester P W. Optimization of the tetrazolium dye (MTT) colorimetric assay for cellular growth and viability [J]. Drug Des Discov: Methods Protocols Methods Mol Biol, 2011, 716: 157-168. |
| [20] | Shyu M H, Kao T C, Yen G C. Oleanolic acid and ursolic acid induce apoptosis in HuH 7 human hepatocellular carcinoma cells through a mitochondrial-dependent pathway and downregulation of XIAP [J]. J Agric Food Chem, 2009, 58(10): 6110-6118. |
 2014, Vol. 45
2014, Vol. 45


