赶黄草为虎耳草科(Saxifragaceae)扯根菜属Penthorum Gronov. ex L.植物扯根菜Penthorum chinense Pursh.的干燥地上部分,又名水泽兰、水杨柳和扯根菜,主产于四川古蔺县。赶黄草具有除湿利水、祛瘀止痛的功效,主治黄疸、经闭、水肿、跌打损伤等症[1]。作为民间传统的药食两用植物[2],由其花、叶、茎加工的具有保健功能的袋泡茶、饮料等已在市场上广泛销售[3]。由单味药材赶黄草水煎液制成的肝苏颗粒,在临床上广泛用于治疗急慢性乙肝、病毒性肝炎、肝纤维化等肝脏疾病[4-6],疗效显著,但赶黄草的药效物质基础尚不明确。本课题组前期实验发现赶黄草水煎液对酒精性脂肪肝和非酒精性脂肪肝具有保护作用[7-8],其醋酸乙酯萃取部分对H2O2诱导的HL7702肝细胞损伤有保护作用,并从中获得了多个结构新颖的木脂素类化合物[9-10]。为进一步明确赶黄草水煎液的物质基础,本实验继续对其醋酸乙酯萃取部分进行分离纯化,获得了9个黄酮类化合物,分别鉴定为6'-羟基-2'-甲氧基二氢查耳酮-4'-O-β-D-吡喃葡萄糖苷(6'-hydroxy-2'-methoxy-dihydrochalcone-4'-O-β-D-glucopyranoside,1)、山柰酚(kaempferol,2)、槲皮素(qercetin,3)、槲皮苷(quercitrin,4)、广寄生苷(avicularin,5)、阿福豆苷(afzelin,6)、绣线菊苷(helicin,7)、乔松素-7-O-[4", 6"-(S)-六羟基联苯二酰基]-β-D-葡萄糖苷(pinocembrin-7-O-[4", 6"-(S)-hexahydroxydiphenoyl]-β-D-glucoside,8)、乔松素-7-O-[3"-O-没食子酰基-4", 6"-(S)-六羟基联苯二酰基]-β-D-葡萄糖苷(pinocembrin-7-O-[3"-O-galloyl-4", 6"-(S)-hexahydroxydiphenoyl]-β-D-glucoside,9)。其中化合物1为新化合物,命名为赶黄草苷,化合物5~7为首次从该属植物中分离得到。
1 仪器与材料Bruker-600、Bruker-500核磁共振波谱仪(Bruker公司);Waters Synapt G2高分辨质谱仪(Waters公司);Agilent 1220型高效液相色谱仪(Agilent公司);D101大孔吸附树脂为成都市科龙化工试剂厂生产;聚酰胺(60~90目)为江苏长丰化工有限公司生产;Búchi Gradient Former B-687中压液相色谱仪(Rp C18,40~60 μm,Welch公司);Sephadex LH-20为瑞典Amershan Pharmacia公司生产;柱色谱硅胶(200~300目)、薄层色谱硅胶GF254均为青岛海洋化工厂生产;GF254硅胶制备薄层板为烟台江友硅胶开发有限公司生产;所用试剂均为分析纯。
赶黄草于2012年7月采自四川古蔺县,经成都中医药大学李敏教授鉴定为虎耳草科扯根菜属植物扯根菜Penthorum chinense Pursh.的干燥地上部分,标本(SGHC-20120725)存放于成都中医药大学中药资源系统研究与开发利用省部共建国家重点实验室培育基地。
2 提取与分离取赶黄草16 kg,用130 L水煎煮3次,每次1 h,合并3次滤液,减压蒸发,浓缩得浸膏1.8 kg。将浓缩所得的浸膏用2 L水悬浮,用8倍体积的醋酸乙酯萃取,将醋酸乙酯萃取物470 g吸附在D101大孔吸附树脂柱上,依次用30%、50%、75%、95%乙醇-水洗脱,每次15 L,回收溶剂得4个洗脱部分A~D。B部分(120 g)经聚酰胺柱色谱,依次用20%、40%、60%、70%、95%乙醇洗脱,60%乙醇组分经凝胶柱色谱、半制备薄层色谱分离得到化合物2(14 mg)、3(20 mg)和4(20 mg);40%乙醇-水组分经凝胶柱色谱、制备薄层色谱和半制备液相色谱分离得到化合物1(15 mg)、5(10 mg)、6(12 mg)和7(13 mg)。C部分(65 g)经硅胶柱色谱分离,二氯甲烷-甲醇(1:0→0:1)梯度洗脱,得到11个组分F1~F11,F7经中压液相色谱、Sephadex LH-20柱色谱和半制备液相色谱反复分离纯化得到化合物8(14 mg)和9(20 mg)。
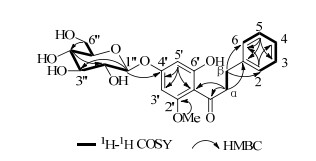
3 结构鉴定化合物1:白色粉末;HR-ESI-MS给出准分子离子峰m/z: 457.147 5 [M+Na]+(C22H26O9Na,计算值为457.147 5),推测其分子式为C22H26O9,不饱和度为10。1H-NMR谱(600 MHz, CD3OD)显示1个单取代苯环质子信号δ 7.26 (2H, t, J=7.2 Hz, H-3, 5), 7.21 (2H, d, J=7.2 Hz, H-2, 6), 7.16 (1H, t, J=7.2 Hz, H-4);1个1, 2, 4, 6-四取代苯环质子信号δ 6.27 (1H, d, J=2.4 Hz, H-5'), 6.21 (1H, d, J=2.4 Hz, H-3');1个芳香甲氧基信号δ3.88 (3H, s, OMe);1个β-葡萄糖基端基质子信号δ 4.98 (1H, d, J=7.2 Hz, H-1"),以及多个连氧亚甲基和次甲基信号(δ 3.35~3.50)(表 1)。13C-NMR谱除了给出与上述基团对应的碳信号外,还显示1个酮羰基信号δC 206.4。结合以上波谱信息,推测化合物1属于二氢查耳酮苷类。化合物1经稀盐酸水解后得到葡萄糖单糖,其显示正旋光性([α]20D +47.4°),且与对照品D-葡萄糖具有相同的TLC行为(MeCN-H2O 8:1),故鉴定为D-葡萄糖[11]。经与已知化合物uvangoletin的波谱数据[12]对比,发现化合物1为uvangoletin的β-D-葡萄糖苷。为进一步确定糖苷化位置,将化合物1的波谱数据与2', 6'-二羟基-二氢查耳酮-4'-O-β-D-吡喃葡萄糖苷相比[13],发现二者的区别在于化合物1的NMR谱中多1个芳香甲氧基信号,且C-2'向低场位移Δδ 4.0,提示化合物1可能为2'-OMe类似物。该推论得到了2D-NMR实验的确证,尤其是在HMBC谱中,H-1"、H-3'、H-5'同时与C-4'相关,OMe-2'与C-2'相关,证明葡萄糖基连接在C-4'位(图 1)。因此,化合物1的结构鉴定为6'-羟基-2'-甲氧基二氢查耳酮-4'-O-β-D-吡喃葡萄糖苷,为新化合物,命名为赶黄草苷。
|
|
表 1 化合物1的1H-NMR和13C-NMR数据(600/150 MHz, CD3OD) Table 1 1H-NMR and 13C-NMR data for compound 1 (600/150 MHz, CD3OD) |
|
图 1 化合物1的结构及主要的1H-1H COSY和HMBC相关信号 Fig.1 Structure, key 1H-1H COSY, and HMBC correlations of compound 1 |
化合物2:黄色粉末;ESI-MS m/z: 309.0 [M+Na]+,分子式为C15H10O6;1H-NMR (500 MHz, CD3OD) δ: 8.09 (2H, d, J=8.5 Hz, H-2', 6'), 6.91 (2H, d, J=8.0 Hz, H-3', 5'), 6.38 (1H, d, J=1.5 Hz, H-8), 6.17 (1H, d, J=1.5 Hz, H-6);13C-NMR (125 MHz, CD3OD) δ: 175.6 (C-4), 164.4 (C-7), 161.7 (C-5), 159.5 (C-4'), 156.4 (C-9), 146.3 (C-2), 136.0 (C-3), 129.9 (C-2', 6'), 122.7 (C-1'), 115.8 (C-3', 5'), 103.8 (C-10), 98.8 (C-6), 94.0 (C-8)。以上数据与文献报道[14]基本一致,故鉴定化合物2为山柰酚。
化合物3:黄色粉末;ESI-MS m/z: 325.0 [M+Na]+,分子式为C15H10O7;1H-NMR (500 MHz, CD3OD) δ: 7.73 (1H, d, J=2.0 Hz, H-2'), 7.64 (1H, dd, J=8.5, 2.0 Hz, H-6'), 6.88 (1H, d, J=8.5 Hz, H-5'), 6.38 (1H, d, J=2.0 Hz, H-8), 6.18 (1H, d, J=2.0 Hz, H-6);13C-NMR (125 MHz, CD3OD) δ: 176.0 (C-4), 164.1 (C-7), 160.9 (C-5), 156.3 (C-9), 147.9 (C-4'), 147.0 (C-2), 145.2 (C-3'), 135.9 (C-3), 122.1 (C-1'), 120.1 (C-6'), 116.4 (C-5'), 115.8 (C-2'), 103.2 (C-10), 98.2 (C-6), 93.5 (C-8)。以上数据与文献报道[15]基本一致,故鉴定化合物3为槲皮素。
化合物4:黄色粉末;ESI-MS m/z: 471.1 [M+Na]+,分子式为C21H20O11;1H-NMR (500 MHz, CD3OD) δ: 7.35 (1H, d, J=2.0 Hz, H-2'), 7.32 (1H, dd, J=8.5, 2.0 Hz, H-6'), 6.92 (1H, d, J=8.5 Hz, H-5'), 6.36 (1H, s, H-8), 6.18 (1H, s, H-6), 5.34 (1H, brs, H-1"), 4.20 (1H, brs, H-2"), 3.34 (1H, m, H-3"), 3.32 (2H, m, H-4", 5"), 0.94 (3H, d, J=6.5 Hz, H-6");13C-NMR (125 MHz, CD3OD) δ: 180.3 (C-4), 166.5 (C-7), 162.1 (C-5), 159.2 (C-2), 158.5 (C-9), 149.8 (C-4'), 146.4 (C-3'), 136.1 (C-3), 122.9 (C-6'), 122.8 (C-1'), 116.9 (C-2'), 116.4 (C-5'), 105.7 (C-10), 103.4 (C-1"), 99.7 (C-6), 94.9 (C-8), 73.0 (C-4"), 72.1 (C-3"), 70.0 (C-2"), 71.9 (C-5"), 17.7 (C-6")。以上数据与文献报道[16]基本一致,故鉴定化合物4为槲皮苷。
化合物5:黄色粉末;ESI-MS m/z: 457.1 [M+Na]+,分子式为C20H18O11;1H-NMR (500 MHz, CD3OD) δ: 7.52 (1H, d, J=1.5 Hz, H-2'), 7.49 (1H, dd, J=8.5, 1.5 Hz, H-6'), 6.90 (1H, d, J=8.5 Hz, H-5'), 6.37 (1H, d, J=2.0 Hz, H-8), 6.19 (1H, d, J=2.0 Hz, H-6), 4.31 (1H, d, J=2.0 Hz, H-1");13C-NMR (125 MHz, CD3OD) δ: 177.9 (C-4), 164.4 (C-7), 161.4 (C-5), 157.0 (C-9), 156.4 (C-2), 148.6 (C-4'), 145.2 (C-3'), 133.6 (C-3), 121.8 (C-6'), 121.2 (C-1'), 115.7 (C-5'), 115.3 (C-2'), 108.1 (C-1"), 104.2 (C-10), 98.7 (C-6), 93.5 (C-8), 86.1 (C-4"), 82.3 (C-2"), 77.3 (C-3"), 60.9 (C-5")。以上数据与文献报道[17]基本一致,故鉴定化合物5为广寄生苷。
化合物6:黄色粉末;ESI-MS m/z: 455.1 [M+Na]+,分子式为C21H20O10;1H-NMR (500 MHz, CD3OD) δ: 7.77 (2H, d, J=8.5 Hz, H-2', 6'), 6.94 (2H, d, J=8.5 Hz, H-3', 5'), 6.37 (1H, s, H-8), 6.19 (1H, s, H-6), 5.37 (1H, s, H-1"), 0.92 (3H, d, J=5.0 Hz, H-6");13C-NMR (125 MHz, CD3OD) δ: 177.9 (C-4), 164.6 (C-7), 161.5 (C-5), 160.2 (C-4'), 157.2 (C-9), 156.9 (C-2), 134.4 (C-3), 130.9 (C-2', 6'), 120.7 (C-1'), 115.6 (C-3', 5'), 104.3 (C-10), 101.8 (C-1"), 99.0 (C-6), 93.9 (C-8), 70.9 (C-3"), 70.5 (C-2"), 71.3 (C-4"), 70.3 (C-5"), 17.7 (C-6")。以上波谱数据与文献报道[18]基本一致,故鉴定化合物6为阿福豆苷。
化合物7:黄色粉末;ESI-MS m/z: 487.1 [M+Na]+,分子式为C21H20O12;1H-NMR (500 MHz, CD3OD) δ: 7.77 (1H, s, H-2'), 7.72 (1H, d, J=8.5 Hz, H-6'), 7.30 (1H, d, J=8.5 Hz, H-5'), 6.38 (1H, s, H-8), 6.15 (1H, s, H-6), 4.91 (1H, d, J=7.5 Hz, H-1"), 3.41~3.94 (6H, m, H-2", 6");13C-NMR (125 MHz, CD3OD) δ: 176.2 (C-4), 164.4 (C-7), 161.0 (C-5), 156.9 (C-9), 146.8 (C-3'), 146.4 (C-4'), 145.8 (C-2), 136.7 (C-3), 126.2 (C-1'), 120.2 (C-6'), 116.3 (C-2'), 115.3 (C-5'), 103.4 (C-10), 101.9 (C-1"), 98.2 (C-6), 93.5 (C-8), 77.0 (C-3"), 76.2 (C-5"), 73.5 (C-2"), 70.1 (C-4"), 61.0 (C-6")。以上波谱数据与文献报道[19]基本一致,故鉴定化合物7为绣线菊苷。
化合物8:黄色固体;ESI-MS m/z: 721.1 [M+H]+,分子式为C35H28O17;1H-NMR (500 MHz, CD3OD) δ: 7.50 (2H, m, H-2', 6'), 7.41 (3H, m, H-3', 4', 5'), 6.69 (1H, s, H-3''''), 6.56 (1H, s, H-3'''), 6.24 (1H, d, J=2.0 Hz, H-8), 6.22 (1H, d, J=2.0 Hz, H-6), 5.47 (1H, dd, J=12.5, 3.0 Hz, H-2), 5.24 (1H, dd, J=13.0, 6.0 Hz, H-6"a), 5.02 (1H, d, J=7.5 Hz, H-1"), 4.84 (1H, t, J=9.0 Hz, H-4"), 4.05 (1H, dd, J=9.0, 6.0 Hz, H-5"), 3.80 (1H, brd, J=13.0 Hz, H-6"b), 3.70 (1H, t, J=9.0 Hz, H-3"), 3.56 (1H, m, H-2"), 3.15 (1H, dd, J=17.0, 12.5 Hz, H-3a), 2.82 (1H, dd, J=17.0, 3.0 Hz, H-3b);13C-NMR (125 MHz, CD3OD) δ: 198.2 (C-4), 169.7 (C-7'''), 169.5 (C-7''''), 166.7 (C-7), 165.2 (C-5), 164.5 (C-9), 145.7 (C-4''', 4''''), 145.1 (C-6''', 6''''), 140.2 (C-1'), 137.5 (C-5''', 5''''), 129.8 (C-3', 4', 5'), 127.5 (C-2', 6'), 126.5 (C-2''', 2''''), 116.8 (C-1''''), 116.9 (C-1'''), 108.7 (C-3'''), 108.5 (C-3''''), 105.1 (C-10), 101.6 (C-1"), 98.1 (C-6), 96.8 (C-8), 80.8 (C-2), 75.6 (C-4"), 75.3 (C-2"), 73.0 (C-3", 5"), 64.4 (C-6"), 44.3 (C-3)。以上波谱数据与文献报道[20]基本一致,故鉴定化合物8为乔松素-7-O-[4", 6"-(S)-六羟基联苯二酰基]-β-D-葡萄糖苷。
化合物9:黄色固体;ESI-MS m/z: 873.2 [M+H]+,分子式为C42H32O21;1H-NMR (500 MHz, CD3OD) δ: 7.50 (2H, m, H-2', 6'), 7.40 (3H, m, H-3', 4', 5'), 7.02 (2H, s, H-2''''', 6'''''), 6.58 (1H, s, H-3''''), 6.47 (1H, s, H-3'''), 6.26 (1H, d, J=2.0 Hz, H-8), 6.24 (1H, d, J=2.0 Hz, H-6), 5.48 (1H, dd, J=13.0, 3.0 Hz, H-2), 5.40 (1H, t, J=9.5 Hz, H-3"), 5.31 (1H, dd, J=13.0, 6.0 Hz, H-6"a), 5.18 (1H, d, J=7.5 Hz, H-1"), 5.05 (1H, t, J=9.5 Hz, H-4"), 4.23 (1H, dd, J=9.5, 6.0 Hz, H-5"), 3.86 (1H, brd, J=13.0 Hz, H-6"b), 3.84 (1H, m, H-2"), 3.14 (1H, dd, J=17.0, 13.0 Hz, H-3a), 2.81 (1H, dd, J=17.0, 3.0 Hz, H-3b);13C-NMR (125 MHz, CD3OD) δ: 198.2 (C-4), 169.6 (C-7'''), 169.3 (C-7''''), 168.1 (C-7'''''), 166.6 (C-7), 165.2 (C-5), 164.5 (C-9), 146.4 (C-3''''', 5'''''), 145.8 (C-4''', 4''''), 144.8 (C-6''', 6''''), 140.2 (C-1'), 139.9 (C-4'''''), 137.6 (C-5''', 5''''), 129.8 (C-3', 4', 5'), 127.5 (C-2', 6'), 126.3 (C-2''''), 126.0 (C-2'''), 121.2 (C-1'''''), 116.7 (C-1''''), 116.5 (C-1'''), 110.7 (C-2''''', 6'''''), 108.7 (C-3''''), 108.4 (C-3'''), 105.2 (C-10), 101.6 (C-1"), 98.2 (C-6), 96.9 (C-8), 80.9 (C-2), 76.0 (C-3"), 73.3 (C-2"), 73.0 (C-5"), 71.4 (C-4"), 64.1 (C-6"), 44.3 (C-3)。以上波谱数据与文献报道[20]基本一致,故鉴定化合物9为乔松素-7-O-[3"-O-没食子酰基-4", 6"-(S)-六羟基联苯二酰基]-β-D-葡萄糖苷。
| [1] | 四川省中药材标准[S].2010. |
| [2] | Zhang T T, Xu X L, Jiang M H, et al. Hepatoprotective function of Penthorum chinense Pursh[J]. Food Funct, 2013, 4(11): 1581–1585. DOI:10.1039/c3fo60245a |
| [3] | 杨玲霞, 朱旭江, 刘婷婷. 赶黄草袋泡茶中黄酮类成分的定量分析[J]. 中国现代中药, 2016, 18(2):219–221. |
| [4] | 贺劲松, 颖俊, 陈亮, 等. 肝苏颗粒治疗慢性乙型肝炎肝纤维化的临床研究[J]. 中西医结合肝病杂志, 2007, 17(3):136–137. |
| [5] | 陈晓蓉, 姚华, 蒋音, 等. 肝苏颗粒治疗慢性乙型肝炎的疗效观察[J]. 中华肝脏病杂志, 2004, 22(1):50. |
| [6] | 张长法, 李子贺, 潘雪飞, 等. 肝苏颗粒治疗非酒精性脂肪性肝炎临床观察[J]. 药物流行病学杂志, 2007, 16(1):5–7. |
| [7] | 肖丽萍, 谢晓芳, 宋洋洋, 等. 赶黄草抗酒精性脂肪肝研究[J]. 中药药理与临床, 2014, 30(3):92–94. |
| [8] | 肖丽萍, 宋洋洋, 周彦希, 等. 赶黄草抗非酒精性脂肪肝的实验研究[J]. 中国实验方剂学杂志, 2014, 20(10):125–129. |
| [9] | He Y C, Peng C, Xie X F, et al. Penchinones A-D, two pairs of cis-trans isomers with rearranged neolignane carbon skeletons from Penthorum chinense[J]. RSC Adv, 2015, 94(5): 76788–76794. |
| [10] | He Y C, Zou Y K, Peng C, et al. Penthorin A and B, two unusual 2, 4'-epoxy-8, 5'-neolignans from Penthorum chinese[J]. Fitoterapia, 2015, 100: 7–10. DOI:10.1016/j.fitote.2014.11.006 |
| [11] | Gan M, Zhang Y, Lin S, et al. Glycosides from the root of Iodes cirrhosa[J]. J Nat Prod, 2008, 71(4): 647–654. DOI:10.1021/np7007329 |
| [12] | Kuo Y C, Yang L M, Lin L C. Isolation and immunomodulatory effect of flavonoids from Syzygium samarangense[J]. Planta Med, 2004, 70(12): 1237–1239. DOI:10.1055/s-2004-835859 |
| [13] | Hegde V R, Pu H Y, Patel M, et al. Two antiviral compounds from the plant Stylogne cauliflora as inhibitors of HCV NS3 protease[J]. Bioorg Med Chem Lett, 2013, 13(17): 2925–2928. |
| [14] | 杜月, 王晓琴, 包保全, 等. 祁州漏芦花化学成分研究[J]. 中草药, 2016, 47(16):2817–2821. |
| [15] | Kim S M, Kang K, Jho E H, et al. Hepatoprotective effect of flavonoid glycosides from Lespedeza cuneata against oxidative stress induced by tert-butyl hyperoxide[J]. Phytother Res, 2011, 25(7): 1011–1017. DOI:10.1002/ptr.v25.7 |
| [16] | Yang N Y, Tao W W, Duan J A, et al. Antithrombotic flavonoids from the faeces of Trogopterus xanthipes[J]. Nat Prod Res, 2010, 19(20): 1843–1849. |
| [17] | Markham K R, Ternai B, Stanley R, et al. Carbon-13 NMR studies of flavonoides-Ⅲ naturally occurring flavonoid glycosides and their acylated derivatives[J]. Tetrahedron, 1978, 34(9): 1389–1397. DOI:10.1016/0040-4020(78)88336-7 |
| [18] | Mourad K. Acylated and non-acylated kaempferol monoglycosides from Platanus acerifolia buds[J]. Phytochemistry, 1990, 29(7): 2295–2297. DOI:10.1016/0031-9422(90)83055-6 |
| [19] | Kwon D J, Bae Y S. Flavonoids from the stem bark of Acer komarovii[J]. Chem Nat Compd, 2013, 49(1): 131–132. DOI:10.1007/s10600-013-0531-2 |
| [20] | Huang Y L, Chen C C, Hsu F L, et al. Two tannins from Phyllanthus tenellus[J]. J Nat Prod, 1998, 61(4): 523–524. DOI:10.1021/np970428k |
 2017, Vol. 48
2017, Vol. 48


