牛樟芝Antrodia cinnamomea T. T. Chang & W. N. Chou为中国台湾道地药材,又名樟芝、樟菇、牛樟菇、红樟芝、血灵芝,属担子菌门菌蔁纲无褶菌目多孔菌科薄孔菌属[1]。牛樟芝生长环境较苛刻,仅见台湾的桃园、苗栗、南投、高雄及屏东等海拔450~2 000 m深山密林中,腐生于台湾保育树种牛樟树Cinnamomum kanehirai Hay树干的中空内部或倒伏树干的表面。牛樟芝在民间应用历史已有200年,台湾原住民认为牛樟芝具有解酒、解食物中毒、止腹泻和止吐作用,可以治疗肝脏病变以及缓解体力透支症状[2]。由于牛樟芝功效显著、产量小且价格昂贵,被誉为“森林中的红宝石”。现代药理研究表明,牛樟芝具有抗肿瘤、保肝、免疫调节、降血压、降胆固醇、抑制血小板凝集等生理活性,自1990年开始逐渐成为台湾研究最热门的抗癌药物及保健品之一。
牛樟芝原为台湾特有的野生真菌,近年来逐渐兴起了人工培养。目前福建、云南、江西、浙江、河南、辽宁等地已开始了大规模培养繁殖。人工培养方法主要有4种,分别为椴木栽培法、固体培养法、液体发酵法、皿培式培养法。不同的培养方法对牛樟芝中化学成分的量和种类有一定影响[3]。其中椴木培养法所得产物与野生牛樟芝成分最为相似,但培养周期较长,未被广泛采用;其他3种培养方法由于周期短、产率高等优势已得到广泛应用。见表 1。
| 表 1 牛樟芝人工培养方法比较 Table 1 Comparison on artificial cultivation methods of A. camphorata |
近年来有关牛樟芝中化学成分及药理作用的研究取得较大进展,现将近5年来研究进展进行综述,为牛樟芝的进一步开发利用提供参考依据。
1 化学成分目前从牛樟芝中分离得到的化学成分已超过200种,包括烷醛、芳香烃、羧酸、脂肪酸酯、腺苷等初级代谢产物,另有精油、多糖、三萜、超氧化物歧化酶、凝集素、微量元素、苯的衍生物、安卓奎诺尔等。其中,三萜、多糖、马来酸衍生物、安卓奎诺尔为牛樟芝的主要活性成分。
1.1 三萜、类固醇及甾体皂苷类迄今从牛樟芝中共发现30多种三萜类化合物,大多从野生或椴木培育的子实体中分离得到,人工培养产物所含三萜类化合物的种类较少。牛樟芝三萜苷元多为四环三萜,分为羊毛脂甾烷型、麦角甾烷型2种。从牛樟芝中分离的三萜及甾体皂苷类化学成分结构见图 1和表 2。
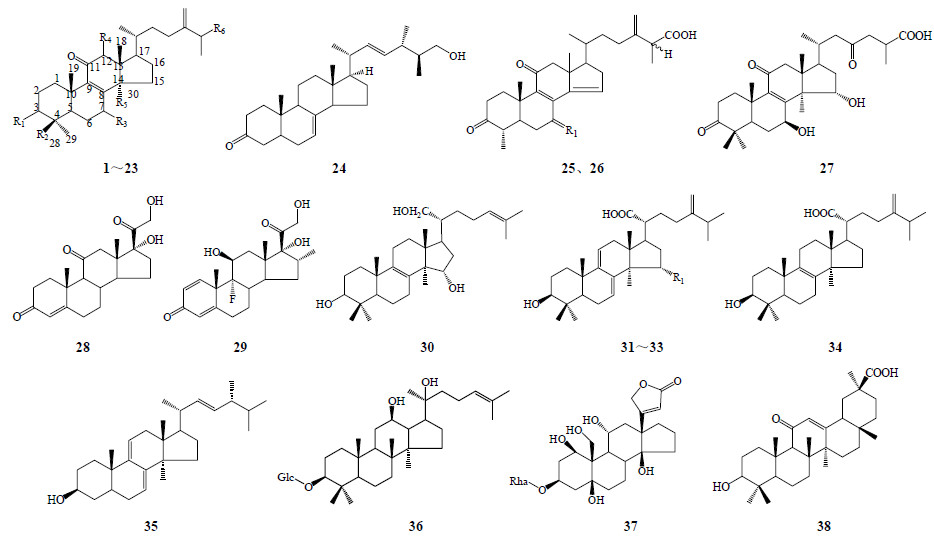 | 图 1 牛樟芝中三萜及甾体皂苷类化合物及母核Fig.1 Structures of triterpenoids and steroidal glycosides in A. cinnamomea |
| 表 2 牛樟芝中发现的三萜和甾体皂苷类成分 Table 2 Triterpenoids and steroidal glycosides in A. cinnamomea |
牛樟芝中的多糖为β-D-葡聚糖结构,具有免疫调节活性。Yang等[9]从菌丝体中分离得到了4种多糖,相对分子质量分别为<5、5~30、30~100、>100。Fa等[10]及Chiu等[11]利用碱提酸沉法并通过琼脂糖凝胶柱色谱从牛樟芝液态发酵菌丝体中分离得到了糖蛋白antrodan,相对分子质量为4.42×105,糖醛酸量(152.6±0.8)mg/g,蛋白质量71.0%。Tsai等[12]通过水提醇沉法从樟芝深层液态发酵菌丝体中制得了水溶性多糖。Cheng等[13]通过木瓜蛋白酶消化,从牛樟芝深层液态发酵菌丝体中分离得由肌糖、海藻糖、半乳糖、葡萄糖组成的硫酸盐多糖。Han等[14]用水提氯仿萃取从牛樟芝菌丝体中制得中性多糖,相对分子质量为1.29×106。
1.3 琥珀酸及马来酸衍生物琥珀酸及马来酸衍生物具有抗炎作用。Wu等[15]从牛樟芝菌丝体甲醇提取物中分离得到马来酸及马来酸酐衍生物,分别为antrocinnamomin A~H(39~46),antrodin A~C(47~49);其中antrocinnamomin A~D为首次分离得到。此外,Nakamura等[16]从牛樟芝菌丝体中分离得到2种琥珀酰亚胺:3R,4S-1-hydroxy-3-isobutyl-4-[4-(3- methyl-2-butenyloxy) phenyl] pyrrolidine-2,5-dione(50)及3R,4R-1-hydroxy-3-isobutyl-4-[4-(3- methyl-2-butenyloxy) phenyl] pyrrolidine-2,5-dione(51)。琥珀酸及马来酸衍生物结构见图 2。
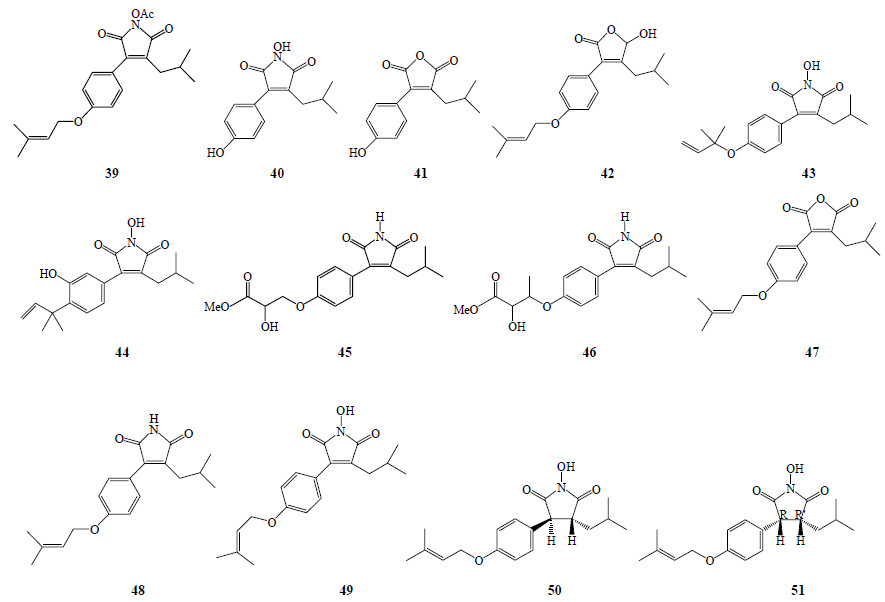 | 图 2 牛樟芝中琥珀酸及马来酸衍生物结构Fig.2 Structures of maleic acid and succinic acid derivatives in A. cinnamomea |
安卓奎诺尔(antroquinonol)是一种仅存在于牛樟芝发酵菌丝体中的泛醌衍生物,属于亲脂型苯醌,具有抗癌作用[17, 18]。安卓奎诺尔及其衍生物结构见图 3。
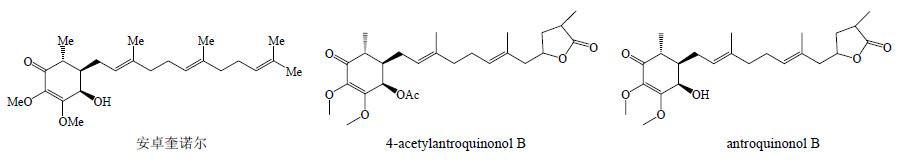 | 图 3 牛樟芝中安卓奎诺尔及其衍生物结构Fig.3 Structures of antroquinonol and its derivatives in A. cinnamomea |
从牛樟芝菌丝体及子实体中分离鉴定了苯醌、苯基、联苯化合物、倍半萜烯及antrocin、γ-dodecalactone[18, 19, 20, 21]。结构见图 4。
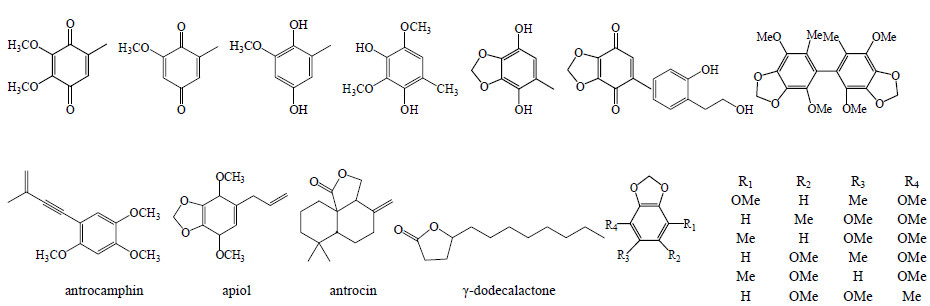 | 图 4 牛樟芝中倍半萜烯和苯的衍生物化学结构Fig.4 Chemical structures of sesquiterpenes and benzenoids in A. cinnamomea |
此外,还从牛樟芝中分离得腺苷有效部位[22],具有活化腺苷受体的生理活性。
2 药理作用牛樟芝被台湾原住民用以解宿醉、解食物中毒、治疗肝脏病变等,由于功效显著逐渐被世人熟知,近年来国内外学者对其进行的大量药理学研究表明,牛樟芝具有抗癌、保肝、抗炎、免疫调节、神经保护等药理作用。
2.1 抗肿瘤作用牛樟芝中的三萜类、多糖、马来酸衍生物、安卓奎诺尔均有抗肿瘤作用,其粗提物在台湾民间被广泛用于治疗肿瘤。牛樟芝的抗肿瘤作用是通过细胞毒作用、抑制癌细胞转移和抑制血管新生作用等机制实现的。
2.1.1 细胞毒作用牛樟芝粗提物对肝癌、乳腺癌、白血病、胰腺癌等癌细胞均具有直接的细胞毒作用。将从牛樟芝中分离的4-acetylantroquinonol B作用于肝癌细胞HepG2,发现该化合物的细胞毒作用呈现量效相关性[22, 23]。从牛樟芝中分离得的8个麦角甾烷型三萜和2个羊毛甾烷三萜及lanosta-8,24-dien- 3β,15α,21-triol具有细胞毒性,IC50值为16.44~77.04 mg/mL[24, 25, 26]。牛樟芝中苯的衍生物可通过钙蛋白介导的细胞通路[27],诱导肝癌细胞HepG2、Hep3B凋亡[28],且不影响正常肝细胞增殖[29]。牛樟芝菌丝体冻干粉乙醇提取物能够抑制头颈癌细胞增殖,且对裸鼠不会引起明显的副反应[30]。进一步研究发现,诱导头颈癌细胞凋亡的活性成分为菌丝体中的antrodin C[31]。
牛樟芝及其活性成分的抗肿瘤活性可能是通过调控p53、p21、p27抑癌基因以及细胞周期基因CDK2、CDK4的表达,使得G1期细胞增多,S期细胞减少实现的[23]。在探索早期抗癌机制时发现,牛樟芝子实体提取物能使肝癌细胞中一系列致癌基因mRNA表达减少,下调磷脂酰肌醇3-激酶/蛋白激酶B(PI3K/Akt)、丝裂原活化蛋白激酶(MAPK)通路,放大细胞自身的凋亡信号[32]。
2.1.2 抑制肿瘤细胞转移研究表明,牛樟芝中的antrodin C能够通过Smad 2/3、β-链蛋白信号通路,抑制转化生长因子-β1(TGF-β1)介导的乳腺癌细胞上皮间质的转化和转移[33]。牛樟芝菌丝体中的糖蛋白antrodan能够抑制肺癌细胞转移[34]。牛樟芝子实体醋酸乙酯萃取物,可通过抑制核因子-κB(NF-κB)的活化,从而抑制肝癌细胞转移[35]。
2.1.3 抑制血管新生牛樟芝多糖具有抗内皮细胞血管新生作用[36]。采用CAM绒毛膜尿囊移植法,发现相对分子质量大于1×105的牛樟芝多糖抑制血管新生效果最好[37]。有报道称,牛樟芝中的硫酸盐多糖抗血管新生作用的强弱与其硫酸盐化程度相关[38]。此外,在与抗肿瘤药阿霉素结合使用时,牛樟芝能显著降低阿霉素导致的心脏毒性、手-足综合征[39]。牛樟芝菌丝体甲醇提取物能使人早幼粒白血病细胞HL-60产生表型功能分化[40]。牛樟芝还可以减少辐射对免疫细胞的损伤,增强辐射对癌细胞的细胞毒作用[41]。
2.2 保肝作用研究表明,牛樟芝具有保肝作用,可以治疗肝炎、肝癌及酒精引起的脂肪肝、肝纤维化等肝脏疾病[42, 43, 44]。从牛樟芝菌丝体中分离的antroquinonol、4-acetylantroquinonol B有抗酒精性肝损伤作用[17, 45]。牛樟芝中的糖蛋白及antrodan能降低脂多糖(LPS)引起的肝细胞氧化损伤、增强肝细胞活性[46, 47],进一步研究发现,牛樟芝多糖的抗氧化作用可能与其修复肝细胞DNA损伤作用有关[48]。另外,牛樟芝中的马来酸及琥珀酸衍生物antrodin A~E也有很强的抑制C型肝炎病毒蛋白酶能力[49],显示出牛樟芝可用于治疗肝癌及HCV感染[50]。
2.3 抗炎及免疫调节作用从牛樟芝中分离的马来酰亚胺衍生物、糖蛋白可以有效降低脂多糖引起的RAW264.7巨噬细胞免疫应答,显示出抗炎作用[50, 51, 52]。该抗炎作用与降低人外周血单核细胞中前免疫活素类肿瘤坏死因子-α(TNF-α)、IL-6及中介物NO、PGE2水平,抑制巨噬细胞中IL-1β、IL-18分泌及NLRP3炎性体,激动MAPK、NF-κB信号通路有关[53, 54]。另外,牛樟芝菌丝体中的methyl antcinate K可以增强树突细胞活性、促进Th2细胞分化,增强免疫应答,具有一定的免疫调节作用[55]。
2.4 抗氧化损伤作用报道显示,牛樟芝具有抗细胞氧化损伤作用。牛樟芝深层液态发酵菌丝体提取物可以清除超氧自由基及DPPH自由基[56]。Antcin C是其抗氧化的主要活性成分[57]。通过比较清除DPPH自由基能力和对超氧化物歧化酶(SOD)活性的影响,显示牛樟芝培养液正己烷、正丁醇、醋酸乙酯部位均有抗氧化能力,其中抗氧化能力最强的部位为醋酸乙酯部位[58]。还可以通过改变培养基中的碳氮比值增强牛樟芝人工培养菌丝体清除自由基的活性[59]。
2.5 神经保护作用研究发现牛樟芝中的多糖、酚类、三萜类、腺苷成分具有一定的神经保护作用。将牛樟芝中硫酸盐多糖、腺苷作用于PC12细胞,发现牛樟芝水提物能调节细胞生存活力[38, 60, 61]。随后将牛樟芝乙醇提取物作用于PC12细胞β淀粉样蛋白(Aβ25-35),发现牛樟芝中多酚类、黄酮类、三萜类、腺苷成分能通过线粒体、腺苷受体途径降低Aβ25-35引起的细胞毒性,从而改善神经退变和记忆功能失调[62]。
2.6 其他作用通过对高脂饲喂仓鼠模型的研究,发现牛樟芝水提物具有调血脂作用[63]。另外,牛樟芝子实体乙醇提取物能通过抑制P-糖蛋白功能影响ABCB1基因多态性,影响药物在体内的跨膜转运及功效发挥[64]。
3 结语牛樟芝起源于中国台湾民间,应用历史已超过200年,用于解毒、解宿醉、治疗肝脏病变、缓解体力透支等。20世纪90年代兴起对牛樟芝的现代药理学的研究。目前,已从牛樟芝中分离得到多种化学成分,其中三萜类、多糖及马来酸衍生物是其主要活性成分。研究显示牛樟芝具有被开发为抗癌及免疫调节药物的潜力,其三萜类及马来酸衍生物可作为抗癌药物;多糖及糖蛋白有望被开发为保肝药物,用于治疗肝炎、肝癌及酒精引起的脂肪肝、肝纤维化等肝脏疾病。
目前,牛樟芝的人工培养技术日渐成熟,未来开发的成本将会降低。但现阶段牛樟芝多作为保健品,较少开发为药物。在中国台湾,牛樟芝子实体保健品生产商有威尔飞、伟翔等,仅有单体antroquinonol被开发为抗癌药,中国大陆地区对牛樟芝的研究开发更少。目前关于牛樟芝粗提物药理活性的报道较多,对单体成分的研究相对匮乏;相关报道亦缺少临床前动物实验及临床试验研究。故今后对牛樟芝中活性成分的分离鉴定、活性成分及部位的药效学、药动学及牛樟芝人工培养产物的质量控制等方面值得深入研究。
| [1] | Lu M, El-Shazly M, Wu T, et al. Recent research and development of Antrodia cinnamomea[J]. Pharmacol Therap, 2013, 139(2):124-156. |
| [2] | 理筱龙. 牛樟芝之简介[A]//首届药用真菌产业发展暨学术研讨会论文集[C]. 南通:中国菌物学会, 2006. |
| [3] | 张东柱. 台湾特有珍贵药用真菌牛樟芝[J]. 食药用菌, 2011, 19(1):33-34. |
| [4] | Yang S W, Shen Y C, Chen C H, et al. Triterpenoids from Antrodia cinnamomea[J]. Phytochemistry, 1996, 41(1):263-267. |
| [5] | Du Y, Wu T, Chang F, et al. Chemical profiling of the cytotoxic triterpenoid-concentrating fraction and characterization of ergostane stereo-isomer ingredients from Antrodia camphorata[J]. J Pharm Biomed Anal, 2012, 58:182-192. |
| [6] | Yang S W, Shen Y C, Chen C H, et al. Steroids and triterpenoids of Antrodia cinnamomea-A fungus parasitic on cinnamomum micranthum[J]. Phytochemistry, 1996, 41(5):1389-1392. |
| [7] | Huang C H, Chen Y J, Lin C C, et al. Antrodia cinnamomea (A. camphorata, Neu chang chih):An exceptional polypore mushroom with potential antitumor and immunomodulatory effects[J]. Curr Topics Nutraceut Res, 2012, 1(10):61-74. |
| [8] | Chen M, Chou C, Chang T, et al. Compound MMH02 possesses toxicity against human cancer cells with sparing of normal monocytes[J]. Int J Gerontol, 2010, 4(4):207-208. |
| [9] | Yang C, Zhou Y, Wang R, et al. Anti-angiogenic effects and mechanisms of polysaccharides from Antrodia cinnamomea with different molecular weights[J]. J Ethnopharmacol, 2009, 123(3):407-412. |
| [10] | Fa K, Yang C, Chen P, et al. Anti-metastatic effects of antrodan, the Antrodia cinnamomea mycelia glycoprotein, in lung carcinoma cells[J]. Int J Biol Macromol, 2015, 74:476-482. |
| [11] | Chiu C, Peng C, Ker Y, et al. Physicochemical characteristics and anti-inflammatory activities of antrodan, a novel glycoprotein isolated from Antrodia cinnamomea Mycelia[J]. Molecules, 2014, 19(1):22-40. |
| [12] | Tsai M, Song T, Shih P, et al. Antioxidant properties of water-soluble polysaccharides from Antrodia cinnamomea in submerged culture[J]. Food Chem, 2007, 104(3):1115-1122. |
| [13] | Cheng J, Huang N, Lur H, et al. Characterization and biological functions of sulfated polysaccharides from sulfated-salt treatment of Antrodia cinnamomea[J]. Process Biochem, 2009, 44(4):453-459. |
| [14] | Han H F, Nakamura N, Zuo F, et al. Protective effects of a neutral polysaccharide isolated from the mycelium of Antrodia cinnamomea on propionibacterium acnes and lipopolysaccharide induced hepatic injury in mice[J]. Pharm Soc Japan, 2006, 54(4):496-500. |
| [15] | Wu M, Cheng M, Wang B, et al.Maleimide and maleic anhydride derivatives from the mycelia of Antrodia cinnamomea and their nitric oxide inhibitory activities in macrophages[J]. J Nat Prod, 2008, 71(7):1258-1261. |
| [16] | Nakamura N, Hirakawa A, Gao J, et al. Five new maleic and succinic acid derivatives from the mycelium of Antrodia camphorata and their cytotoxic effects on LLC tumor cell line[J]. J Nat Prod, 2004, 67(1):46-48. |
| [17] | Kumar K J S, Chu F, Hsieh H, et al. Antroquinonol from ethanolic extract of mycelium of Antrodia cinnamomea protects hepatic cells from ethanol-induced oxidative stress through Nrf-2 activation[J]. J Ethnopharmacol, 2011, 136(1):168-177. |
| [18] | Lien H, Chiu C, Chen C, et al. Comparison of the apoptotic effects of supercritical fluid extracts of Antrodia cinnamomea mycelia on hepatocellular carcinoma cells[J]. Molecules, 2014, 19(7):9033-9050. |
| [19] | Chiang H C, Wu D P, Cherng I W, et al. A sesquiterpene lactone, phenyl and biphenyl compounds from Antrodia cinnamomea[J]. Phytochemistry, 1995, 39(3):613-616. |
| [20] | Shao Y Y, Chen C C, Wang H Y, et al. Chemical constituents of Antrodia camphorata submerged whole broth[J]. Nat Prod Res, 2008, 13(22):1151-1157. |
| [21] | Lin T, Chen C, Chien S, et al. Metabolite profiles for Antrodia cinnamomea fruiting bodies harvested at different culture ages and from different wood substrates[J]. J Agric Food Chem, 2011, 59(14):7626-7635. |
| [22] | Lin Y, Pan J, Liu R H, et al. The 4-acetylantroquinonol B isolated from mycelium of Antrodia cinnamomea inhibits proliferation of hepatoma cells[J]. J Sci Food Agric, 2010, 90(10):1739-1744. |
| [23] | Lin Y, Chiang B. 4-Acetylantroquinonol B isolated from Antrodia cinnamomea arrests proliferation of human hepatocellular carcinoma HepG2 cell by affecting p53, p21 and p27 levels[J]. J Agric Food Chem, 2011, 59(16):8625-8631. |
| [24] | Du Y C, Wu T Y, Chang F R, et al. Chemical profiling of the cytotoxic triterpenoid-concentrating fraction and characterization of ergostane stereo-isomer ingredients from Antrodia camphorata[J]. J Pharm Biomed Anal, 2012, 58:182-192. |
| [25] | Chen Y, Chou C, Chang T. Compound MMH01 possesses toxicity against human leukemia and pancreatic cancer cells[J]. Toxicol In Vitro, 2009, 23(3):418-424. |
| [26] | Chen M J, Chou C J, Chang T T, et al. Compound MMH02 possesses toxicity against human cancer cells with sparing of normal monocytes[J]. Int J Gerontol, 2010, 4(4):207-208. |
| [27] | Kuo P L, Hsu Y L, Cho C Y, et al. Apoptotic effects of Antrodia cinnamomea fruiting bodies extract are mediated through calcium and calpain-dependent pathways in Hep 3B cells[J]. Food Chem Toxicol, 2006, 44(8):1316-1326. |
| [28] | Lien H M, Chiu C H, Chen C C, et al. Comparison of the apoptotic effects of supercritical fluid extracts of Antrodia cinnamomea mycelia on hepatocellular carcinoma cells[J]. Molecules, 2014, 19(7):9033-9050. |
| [29] | Chen Y S, Pan J H, Chiang B H, et al. Ethanolic extracts of Antrodia cinnamomea mycelia fermented at varied times and scales have differential effects on hepatoma cells and normal primary hepatocytes[J]. J Food Sci, 2008, 73(7):179-185. |
| [30] | Chang C W, Chen Y S, Chen C C, et al. Lyophilized particles and ethanolic extracts of Antrodia cinnamomea mycelia suppress the tumorigenicity of head and neck cancer cells in vivo[J]. Biol Med, 2014, 4(4):37-41. |
| [31] | Chang C W, Chen C C, Wu M J, et al. Active component of Antrodia cinnamomea mycelia targeting head and neck cancer initiating cells through exaggerated autophagic cell death[J]. Evid Based Complement Alternat Med, 2013(2013):1-15. |
| [32] | Chen Y J, Thang M W C, Chan Y T, et al. Global assessment of Antrodia cinnamomea-induced microRNA alterations in hepatocarcinoma cells[J]. PLoS One, 2013, 8(12):e82751. |
| [33] | Kumar K J S, Vani M G, Chueh P J, et al. Antrodin C inhibits epithelial-to-mesenchymal transition and metastasis of breast cancer cells via suppression of Smad2/3 and β-catenin signaling pathways[J]. PLoS One, 2015, 10(2):e0117111. |
| [34] | Fa K N, Yang C M, Chen P C, et al. Anti-metastatic effects of antrodan, the Antrodia cinnamomea mycelia glycoprotein, in lung carcinoma cells[J]. Int J Biol Macromol, 2015, 2015(74):476-482. |
| [35] | Hsu Y L, Kuo P L, Cho C Y, et al. Antrodia cinnamomea fruiting bodies extract suppresses the invasive potential of human liver cancer cell line PLC/PRF/5 through inhibition of nuclear factor κB pathway[J]. Food Chem Toxicol, 2007, 45(7):1249-1257. |
| [36] | Cheng J J, Huang N K, Chang T T, et al. Study for anti-angiogenic activities of polysaccharides isolated from Antrodia cinnamomea in endothelial cells[J]. Life Sci, 2005, 76(26):3029-3042. |
| [37] | Yang C M, Zhou Y J, Wang R J, et al. Anti-angiogenic effects and mechanisms of polysaccharides from Antrodia cinnamomea with different molecular weights[J]. J Ethnopharmacol, 2009, 123(3):407-412. |
| [38] | Cheng J J, Huang N K, Lur H S, et al. Characterization and biological functions of sulfated polysaccharides from sulfated-salt treatment of Antrodia cinnamomea[J]. Process Biochem, 2009, 44(4):453-459. |
| [39] | Sheu M T, Jhan H J, Hsieh C M, et al. Efficacy of antioxidants as a complementary and alternative medicine (CAM) in combination with the chemotherapeutic agent doxorubicin[J]. Integr Cancer Ther, 2015, 14(2):184-195. |
| [40] | Wen C L, Teng C L, Chiang C H, et al. Methanol extract of Antrodia cinnamomea mycelia induces phenotypic and functional differentiation of HL60 into monocyte-like cells via an ERK/CEBP-β signaling pathway[J]. Phytomedicine, 2012, 19(5):424-435. |
| [41] | Cheng P C, Huang C C, Chiang P F, et al. Radio protective effects of Antrodia cinnamomea are enhanced on immune cells and inhibited on cancer cells[J]. Int J Radia Biol, 2014, 90(10):841-852. |
| [42] | Yue P Y K, Wong Y Y, Wong K Y K, et al. Current evidence for the hepatoprotective activities of the medicinal mushroom Antrodia cinnamomea[J]. Chin Med, 2013, 8(1):21-27. |
| [43] | Chen Y R, Chang K T, Tsai M J, et al. Antrodia cinnamomea profoundly exalted the reversion of activated hepatic stellate cells by the alteration of cellular proteins[J]. Food Chem Toxicol, 2014, 69:150-162. |
| [44] | Ho Y C, Lin M T, Duan K J, et al. The hepatoprotective activity against ethanol-induced cytotoxicity by aqueous extract of Antrodia cinnamomea[J]. J Chin Institute Chem Eng, 2008, 39(5):441-447. |
| [45] | Wang H C, Chu F H, Chien S C, et al. Establishment of the metabolite profile for an Antrodia cinnamomea health food product and investigation of its chemoprevention activity[J]. J Agric Food Chem, 2013, 61(36):8556-8564. |
| [46] | Ker Y B, Peng C C, Chang W L, et al. Hepatoprotective bioactivity of the glycoprotein, antrodan, isolated from Antrodia cinnamomea mycelia[J]. PLoS One, 2014, 9(4):e93191. |
| [47] | Han H F, Nakamura N, Zuo F, et al. Protective effects of a neutral polysaccharide isolated from the mycelium of Antrodia cinnamomea on propionibacterium acnes and lipopolysaccharide induced hepatic injury in mice[J]. Chem Pharm Bull, 2006, 54(4):496-500. |
| [48] | Tsai M C, Song T Y, Shih P H, et al. Antioxidant properties of water-soluble polysaccharides from Antrodia cinnamomea in submerged culture[J]. Food Chem, 2007, 104(3):1115-1122. |
| [49] | Phuong D T, Ma C M, Hattori M, et al. Inhibitory effects of antrodins A-E from Antrodia cinnamomea and their metabolites on hepatitis C virus protease[J]. Chem Biodivers, 2009, 23(4):582-584. |
| [50] | Chiu C H, Peng C C, Ker Y B, et al. Physicochemical characteristics and anti-inflammatory activities of antrodan, a novel glycoprotein isolated from Antrodia cinnamomea mycelia[J]. Molecules, 2014, 19(1):22-40. |
| [51] | Wu M D, Cheng M J, Yech Y J, et al. Inhibitory effects of maleimide derivatives from the mycelia of the fungus Antrodia cinnamomea BCRC 36799 on nitric oxide production in lipopolysaccharide (LPS) activated RAW264.7 macrophages[J]. Chem Biodivers, 2013, 10(3):434-441. |
| [52] | Wu M D, Cheng M J, Wang B C, et al. Maleimide and maleic anhydride derivatives from the mycelia of Antrodia cinnamomea and their nitric oxide inhibitory activities in macrophages[J]. J Nat Prod, 2008, 71(7):1258-1261. |
| [53] | Wen C L, Chang C C, Huang S S, et al. Anti-inflammatory effects of methanol extract of Antrodia cinnamomea mycelia both in vitro and in vivo[J]. J Ethnopharmacol, 2011, 137(1):575-584. |
| [54] | Huang T T, Wu S P, Chong K Y, et al. The medicinal fungus Antrodia cinnamomea suppresses inflammation by inhibiting the NLRP3 inflammasome[J]. J Ethnopharmacol, 2014, 155(1):154-164. |
| [55] | Yu Y L, Chen I H, Shen K Y, et al. A triterpenoid methyl antcinate K isolated from Antrodia cinnamomea promotes dendritic cell activation and Th2 differentiation[J]. Eur J Immunol, 2009, 39(9):2482-2491. |
| [56] | Lin E N S, Yang C T, Chou H J U, et al. Screening of antioxidant activities by the edible basidiomycete Antrodia cinnamomea strains in submerged culture[J]. J Food Biochem, 2010, 34(6):1141-1156. |
| [57] | Gokila Vani M, Kumar K J, Liao J W, et al. Antcin C from Antrodia cinnamomea protects liver cells against free radical-induced oxidative stress and apoptosis in vitro and in vivo through Nrf2-dependent mechanism[J]. Evid Based Complement Alternat Med, 2013, 2013:1-17. |
| [58] | Wu M D, Cheng M J, Wang W Y, et al. Antioxidant activities of extracts and metabolites isolated from the fungus Antrodia cinnamomea[J]. Nat Prod Res, 2011, 25(16):1488-1496. |
| [59] | Kuo J T, Lin E N, Yang C T. Effect of cultivating conditions on the superoxide and free radical-scavenging activities of Antrodia cinnamomea[J]. J Food Biochem, 2011, 35(5):1493-1500. |
| [60] | Lu M K, Cheng J J, Lai W L, et al. Adenosine as an active component of Antrodia cinnamomea that prevents rat PC12 cells from serum deprivation-induced apoptosis through the activation of adenosine A2A receptors[J]. Life Sci, 2006, 79(3):252-258. |
| [61] | Lu M K, Cheng J J, Lai W L, et al. Fermented Antrodia cinnamomea extract protects rat PC12 cells from serum deprivation-induced apoptosis:The role of the MAPK family[J]. J Agric Food Chem, 2008, 56(3):865-874. |
| [62] | Chang C H, Wang H E, Liaw P Y, et al. Antrodia cinnamomea exhibits a potent neuro-protective effect in the PC12 cell-Aβ 25-35 model-pharmacologically through adenosine receptors and mitochondrial pathway[J]. Planta Med, 2012, 78(17):1813-1823. |
| [63] | Lai M N, Ko H J, Ng L T. Hypolipidemic effects of Antrodia cinnamomea extracts in high-fat diet-fed hamsters[J]. Food Biochem, 2012, 36(2):233-239. |
| [64] | Sheu M J, Teng Y N, Chen Y Y, et al. The functional influences of common ABCB1 genetic variants on the inhibition of P-glycoprotein by Antrodia cinnamomea extracts[J]. PLoS One, 2014, 2(9):1-11. |
 2016, Vol. 47
2016, Vol. 47


