2. 中国科学院上海药物研究所 新药研究国家重点实验室, 上海 201203
2. State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China
圆盘肉芝软珊瑚Sarcophyton trocheliophorum Marenzeller属于腔肠动物门(Coelenterata)珊瑚虫纲(Anthozoa)八放珊瑚亚纲(Octocorallia)海鸡冠目(Alcyonacea)软珊瑚科(Alcyoniidae)肉芝软珊瑚属Sarcophyton。这种软珊瑚分布广泛,在中国南海、日本冲绳、澳大利亚大堡礁等不同纬度的海域都可见到其踪影[1]。虽然圆盘肉芝软珊瑚肉质柔软且厚实,但很少看到软体动物等捕食者攀附其上,这说明其自身能分泌一些具有特殊生物活性的次级代谢产物来抵御捕食者。这种现象引起了世界各国的科学家的兴趣,对该种软珊瑚进行了一系列研究,从中发现了许多结构新颖并具有显著生物活性的次级代谢产物,包括倍半萜、二萜、甾体、前列腺素类似物、γ-丁内酯等,且生物活性广泛,涵盖神经保护、抗肿瘤、抗捕食、抗菌等[1-2]。
为了开发利用我国的海洋生物资源,从中寻找更多具有生物活性及药用前景的海洋天然产物[3-7],本课题组对采自我国南海的圆盘肉芝软珊瑚的化学成分开展了前期研究,从中发现了西松烷型、capnosane型、sarsolenane型和新骨架等多种新颖结构类型的二萜,而且其中一些二萜具有抑制人蛋白酪氨酸酶(PTP1B)、抗菌等多种显著的生物活性[8-11]。鉴于此,本实验采用1H-NMR导向分离法[12]对该种软珊瑚的化学成分开展研究,从中发现了9个化合物(图 1),分别鉴定为瑞士松烯A(cembrene A,1)、(E, E, E)-7, 8-epoxy-1-isopropyl-4, 8, 12-trimethyl-cyclotetradeca-1, 3, 11-triene(2)、sarcophytonolide A(3)、deacetylemblide(4)、4Z, 12Z, 14E-sarcophytolide(5)、sarcrassin D(6)、emblide(7)、(4Z, 8S, 9R, 12E, 14E)-9-hydroxy-1-isopropyl-8, 12-dimethyloxabicyclo [9.3.2]-hexadeca-4, 12, 14-trien-18-one(8)、β-榄香烯(β-elemene,9)。其中化合物1~8为二萜类,化合物9为倍半萜类。化合物4是1个新的天然产物,并首次报道其13C-NMR数据。化合物1、3、5、8为首次从该种软珊湖中分离得到。生物活性研究表明,化合物5对PTP1B有中等强度的抑制作用(IC50=15.4 μmol/L)。
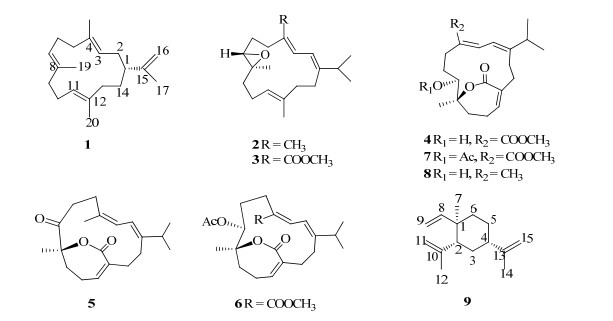
|
图 1 化合物1~9的结构 Fig.1 Structures of compounds 1-9 |
1 仪器与材料
Bruker DRX-400核磁共振仪(德国Bruker公司);Finnigan-MAT-95型质谱仪(美国Finnigan公司);Q-TOF Micro LC-MS质谱仪(美国Waters公司);Shimadzu LC-6AD制备液相色谱仪(日本岛津制作所);Shimadzu SPD-20A紫外检测器(日本岛津制作所);Sephadex LH-20(瑞典Amersham Biosciences公司);柱色谱硅胶(200~300、300~400目,青岛海洋化工有限公司);GF254薄层色谱硅胶板(烟台江友硅胶开发有限公司);色谱级甲醇、乙腈为DiKMA有限公司产品;其他有机试剂均为分析纯,购自国药集团化学试剂有限公司。
实验材料于2011年9月采自我国海南亚龙湾海域,采集后立即冷冻备用。由中国科学院南海海洋研究所的黄晖教授鉴定为中国南海圆盘肉芝软珊瑚Sarcophyton trocheliophorum Marenzeller。样品标本(YAL-6)保存在中国科学院上海药物研究所国家新药研究重点实验室。
2 提取与分离冷冻的软珊瑚样品YAL-6(干质量301 g)切碎后在室温下用丙酮超声提取5次,每次15 min。将提取液减压浓缩除去有机溶剂,浓缩物悬浮于水中,用等体积的乙醚及正丁醇分别萃取5次,有机相萃取液经减压浓缩分别得到乙醚粗浸膏3.5 g及正丁醇浸膏1.9 g。乙醚浸膏经硅胶(200~300目)柱色谱,石油醚-乙醚(乙醚0→100%)梯度洗脱,分为A~U共21个部分。B和C经HPLC分离(甲醇-水100:0,2.0 mL/min),分别得到化合物1(1.3 mg)和9(17.3 mg)。H和J经硅胶(200~300目)柱色谱(分离条件分别为石油醚-乙醚25:1、石油醚-丙酮30:1)分离得到化合物2(13.8 mg)和3(23.0 mg)。N经过凝胶Sephadex LH-20柱色谱(石油醚-氯仿-甲醇2:1:1)和硅胶(200~300目)柱色谱(石油醚-丙酮10:1)分离得到2个亚组分N1和N2。N2经HPLC(乙腈-水70:30,2.0 mL/min)分离得到化合物5(12.0 mg)。O经过凝胶Sephadex LH-20柱色谱(氯仿-甲醇1:1)、硅胶(300~400目)柱色谱(石油醚-乙醚2:1、1:1)、HPLC(乙腈-水80:20,2.0 mL/min)分离得到化合物6(26.3 mg)和7(94.7 mg);P经过凝胶Sephadex LH-20柱色谱(石油醚-氯仿-甲醇2:1:1)、HPLC(甲醇-水75:25,2.0 mL/min)分离得到化合物8(1.1 mg);Q经过凝胶Sephadex LH-20柱色谱(石油醚-氯仿-甲醇2:1:1)、硅胶(300~400目)柱色谱、HPLC(乙腈-水53:47,2.0 mL/min)分离得到化合物4(7.5 mg)。
3 结构鉴定化合物1:无色油状物,分子式为C20H32。EI-MS m/z: 272 [M]+。1H-NMR (400 MHz, CDCl3) δ: 5.05 (1H, t, J=6.7 Hz, H-3), 5.19 (1H, t, J=6.9 Hz, H-7), 4.98 (1H, t, J=6.8 Hz, H-11), 4.71 (1H, brs, H-16a), 4.65 (1H, brs, H-16b), 1.66 (3H, s, H-17), 1.55 (3H, s, H-18), 1.59 (3H, s, H-19), 1.56 (3H, s, H-20);13C-NMR (100 MHz, CDCl3) δ: 46.0 (C-1), 32.4 (C-2), 121.9 (C-3), 134.0 (C-4), 39.0 (C-5), 24.9 (C-6), 124.1 (C-7), 134.8 (C-8), 39.5 (C-9), 23.8 (C-10), 126.0 (C-11), 133.5 (C-12), 34.0 (C-13), 28.2 (C-14), 149.2 (C-15), 110.2 (C-16), 19.4 (C-17), 18.0 (C-18), 15.2 (C-19), 15.5 (C-20)。以上数据与文献报道[13]基本一致,故鉴定化合物1为瑞士松烯A。
化合物2:无色油状物,分子式为C20H32O。EI-MS m/z: 288 [M]+。1H-NMR (400 MHz, CDCl3) δ: 6.03 (1H, d, J=11.0 Hz, H-2), 5.95 (1H, d, J=11.2 Hz, H-3), 2.84 (1H, t, J=5.6 Hz, H-7), 5.06 (1H, t, J=6.7 Hz, H-11), 1.05 (3H, d, J=6.8 Hz, H-16), 1.04 (3H, d, J=7.0 Hz, H-17), 1.74 (3H, s, H-18), 1.25 (3H, s, H-19), 1.58 (3H, s, H-20);13C-NMR (100 MHz, CDCl3) δ: 148.1 (C-1), 118.4 (C-2), 121.4 (C-3), 134.2 (C-4), 35.8 (C-5), 37.4 (C-6), 61.6 (C-7), 60.0 (C-8), 25.7 (C-9), 22.5 (C-10), 125.5 (C-11), 135.6 (C-12), 39.5 (C-13), 28.2 (C-14), 34.9 (C-15), 22.3 (C-16), 22.1 (C-17), 17.1 (C-18), 17.8 (C-19), 17.0 (C-20)。以上数据与文献报道[14]基本一致,故鉴定化合物2为(E, E, E)-7, 8-epoxy-1-isopropyl-4, 8, 12-trimethylcyclotetradeca-1, 3, 11-triene。
化合物3:无色油状物,分子式为C21H32O3。ESI-MS m/z: 355.1 [M+Na]+。1H-NMR (400 MHz, CDCl3) δ: 7.00 (1H, d, J=11.8 Hz, H-2), 6.66 (1H, d, J=11.8 Hz, H-3), 2.78 (1H, m, H-7), 5.02 (1H, t, J=6.2 Hz, H-11), 1.08 (3H, d, J=6.6 Hz, H-16), 1.09 (3H, d, J=6.6 Hz, H-17), 1.20 (3H, s, H-19), 1.60 (3H, s, H-20), 3.75 (3H, s, 18-COOMe);13C-NMR (100 MHz, CDCl3) δ: 157.7 (C-1), 120.1 (C-2), 138.0 (C-3), 125.3 (C-4), 31.5 (C-5), 26.6 (C-6), 60.4 (C-7), 60.6 (C-8), 36.4 (C-9), 22.0 (C-10), 127.2 (C-11), 134.0 (C-12), 38.3 (C-13), 28.4 (C-14), 34.8 (C-15), 22.2 (C-16), 22.0 (C-17), 168.0 (C-18), 18.2 (C-19), 17.3 (C-20), 51.3 (18-COOMe)。以上数据与文献报道[15]基本一致,故鉴定化合物3为sarcophytonolide A。
化合物4:无色油状物,分子式为C21H30O5。ESI-MS m/z: 385.3 [M+Na]+。1H-NMR (400 MHz, CDCl3) δ: 6.94 (1H, d, J=11.6 Hz, H-2), 6.25 (1H, d, J=11.6 Hz, H-3), 4.12 (1H, d, J=11.8 Hz, H-7), 6.08 (1H, brs, H-11), 1.08 (3H, d, J=6.9 Hz, H-16), 1.11 (3H, d, J=6.9 Hz, H-17), 1.40 (3H, s, H-19), 3.79 (3H, s, 18-COOMe)。以上数据与文献报道[16]基本一致,故鉴定化合物4为deacetylemblide,并首次报道其13C-NMR数据。13C-NMR (100 MHz, CDCl3) δ: 155.9 (C-1), 120.0 (C-2), 135.3 (C-3), 124.8 (C-4), 25.6 (C-5), 27.5 (C-6), 65.3 (C-7), 83.3 (C-8), 34.4 (C-9), 27.0 (C-10), 142.3 (C-11), 132.1 (C-12), 36.7 (C-13), 27.7 (C-14), 35.3 (C-15), 21.8 (C-16), 22.1 (C-17), 170.0 (C-18), 22.9 (C-19), 166.6 (C-20), 51.6 (18-COOMe)。
化合物5:无色油状物,分子式为C20H28O3。ESI-MS m/z: 339.3 [M+Na]+。1H-NMR (400 MHz, CDCl3) δ: 6.26 (1H, brt, J=5.0 Hz, H-5), 5.76 (1H, d, J=9.3 Hz, H-13), 5.80 (1H, d, J=9.3 Hz, H-14), 0.97 (3H, d, J=6.9 Hz, H-16), 1.09 (3H, d, J=6.8 Hz, H-17), 1.45 (3H, s, H-19), 1.86 (3H, s, H-20);13C-NMR (100 MHz, CDCl3) δ: 145.9 (C-1), 25.6 (C-2), 27.5 (C-3), 132.0 (C-4), 143.6 (C-5), 34.0 (C-6), 32.8 (C-7), 87.2 (C-8), 211.4 (C-9), 31.8 (C-10), 33.9 (C-11), 137.7 (C-12), 122.5 (C-13), 119.5 (C-14), 31.2 (C-15), 20.5 (C-16), 23.0 (C-17), 166.8 (C-18), 29.4 (C-19), 26.7 (C-20)。以上数据与文献报道[17]基本一致,故鉴定化合物5为4Z, 12Z, 14E-sarcophytolide。
化合物6:无色油状物,分子式为C23H32O6。ESI-MS m/z: 427.2 [M+Na]+。1H-NMR (400 MHz, CDCl3) δ: 5.91 (1H, d, J=8.3 Hz, H-2), 7.51 (1H, d, J=8.3 Hz, H-3), 5.23 (1H, brd, J=10.8 Hz, H-7), 6.29 (1H, t, J=3.5 Hz, H-11), 1.06 (3H, d, J=6.6 Hz, H-16), 0.98 (3H, d, J=6.8 Hz, H-17), 1.35 (3H, s, H-19), 3.70 (3H, s, 18-COOMe), 2.02 (3H, s, 7-COMe);13C-NMR (100 MHz, CDCl3) δ: 155.3 (C-1), 119.1 (C-2), 137.0 (C-3), 128.9 (C-4), 20.6 (C-5), 25.8 (C-6), 68.1 (C-7), 82.0 (C-8), 34.2 (C-9), 27.3 (C-10), 141.9 (C-11), 132.1 (C-12), 34.2 (C-13), 32.9 (C-14), 31.0 (C-15), 19.8 (C-16), 22.9 (C-17), 168.6 (C-18), 23.9 (C-19), 166.6 (C-20), 51.8 (18-COOMe), 169.8 (7-COMe), 21.0 (7-COMe)。以上数据与文献报道[18]基本一致,故鉴定化合物6为sarcrassin D。
化合物7:无色油状物,分子式为C23H32O6。ESI-MS m/z: 427.2 [M+Na]+。1H-NMR (400 MHz, CDCl3) δ: 7.15 (1H, d, J=11.9 Hz, H-2), 6.26 (1H, d, J=11.8 Hz, H-3), 5.39 (1H, dd, J=9.0, 1.7 Hz, H-7), 6.12 (1H, t, J=3.6 Hz, H-11), 1.12 (3H, d, J=6.8 Hz, H-16), 1.07 (3H, d, J=6.8 Hz, H-17), 1.47 (3H, s, H-19), 3.75 (3H, s, 18-COOMe), 2.03 (3H, s, 7-COMe);13C-NMR (100 MHz, CDCl3) δ: 155.3 (C-1), 120.8 (C-2), 135.4 (C-3), 124.3 (C-4), 26.2 (C-5), 25.1 (C-6), 68.1 (C-7), 82.4 (C-8), 34.5 (C-9), 27.2 (C-10), 142.3 (C-11), 132.0 (C-12), 37.1 (C-13), 27.1 (C-14), 35.9 (C-15), 22.7 (C-16), 21.9 (C-17), 168.3 (C-18), 23.7 (C-19), 166.4 (C-20), 51.3 (18-COOMe), 169.7 (7-COMe), 20.9 (7-COMe)。以上数据与文献报道[16]基本一致,故鉴定化合物7为emblide。
化合物8:无色油状物,分子式为C20H30O3。ESI-MS m/z: 341.2 [M+Na]+。1H-NMR (400 MHz, CDCl3) δ: 6.11 (1H, brs, H-5), 4.29 (1H, d, J=10.5 Hz, H-9), 5.56 (1H, d, J=11.5 Hz, H-13), 6.13 (1H, d, J=11.4 Hz, H-14), 1.08 (3H, d, J=6.8 Hz, H-16), 1.04 (3H, d, J=6.8 Hz, H-17), 1.38 (3H, s, H-19), 1.77 (3H, s, H-20);13C-NMR (100 MHz, CDCl3) δ: 146.1 (C-1), 27.1 (C-2), 27.5 (C-3), 133.4 (C-4), 140.2 (C-5), 34.5 (C-6), 37.4 (C-7), 83.4 (C-8), 66.3 (C-9), 26.8 (C-10), 31.4 (C-11), 132.2 (C-12), 121.0 (C-13), 118.7 (C-14), 35.7 (C-15), 22.1 (C-16), 22.6 (C-17), 166.9 (C-18), 21.8 (C-19), 19.0 (C-20)。以上数据与文献报道[17]基本一致,故鉴定化合物8为(4Z, 8S, 9R, 12E, 14E)-9-hydroxy-1-isopropyl-8, 12-dimethyloxabicyclo [9.3.2]-hexadeca-4, 12, 14-trien-18-one。
化合物9:无色油状物,分子式为C15H24。EI-MS m/z: 204 [M]+。1H-NMR (400 MHz, CDCl3) δ: 1.01 (3H, s, H-7), 5.83 (1H, dd, J=17.0, 12.0 Hz, H-8), 4.90 (1H, d, J=12.0 Hz, H-9a), 4.91 (1H, d, J=16.7 Hz, H-9b), 4.60 (1H, s, H-11a), 4.72 (1H, s, H-11b), 1.72 (3H, s, H-12), 1.75 (3H, s, H-14), 4.71 (1H, s, H-15a), 4.83 (1H, s, H-15b);13C-NMR (100 MHz, CDCl3) δ: 39.8 (C-1), 52.7 (C-2), 32.9 (C-3), 45.7 (C-4), 26.8 (C-5), 39.9 (C-6), 16.6 (C-7), 150.3 (C-8), 112.0 (C-9), 147.7 (C-10), 109.2 (C-11), 24.8 (C-12), 150.4 (C-13), 21.1 (C-14), 108.2 (C-15)。以上数据与文献报道[19-20]基本一致,故鉴定化合物9为β-榄香烯。
4 生物活性大量实验表明,PTP1B是治疗2型糖尿病和肥胖症的潜在靶点,寻找其抑制剂是防治2型糖尿病和肥胖症的重要研究方向之一[21]。与前期研究所采用的方法相同[8-9],化合物1~9开展了PTP1B抑制活性的筛选实验。结果表明,化合物5对PTP1B具有中等强度的抑制作用(IC50=15.4 μmol/L),而其他化合物无明显的生物活性。初步分析化合物4~8的结构及生物活性,推测西松烷结构中7位羰基的存在可能加强该类二萜的PTP1B抑制活性。抗菌、抗炎、神经保护等其他生物活性研究还在进行中。
5 讨论本实验分离得到的9个化合物中,8个为二萜,1个为倍半萜。其中,二萜类化合物4是1个新的天然产物,并首次报道其13C-NMR数据。二萜类化合物1、3、5、8都是首次从该种软珊湖中分离得到。通过与前期研究工作的对比,发现采用1H-NMR导向分离法对圆盘肉芝软珊瑚开展的每一次研究工作都会得到不同的新颖结构类型的活性次级代谢产物,这表明该种软珊瑚值得深入研究。生物活性研究结果表明,化合物5对PTP1B具有中等强度的抑制作用,IC50值为15.4 μmol/L。上述研究结果进一步丰富了圆盘肉芝软珊瑚的化学成分及生物学活性多样性,并为2型糖尿病潜在治疗药物--PTP1B抑制剂的研发提供一些参考。
| [1] | Liang L F, Guo Y W. Terpenes from the soft corals of the genus Sarcophyton:chemistry and biological activities[J]. Chem Biodivers, 2013, 10(12): 2161–2196. DOI:10.1002/cbdv.v10.12 |
| [2] | Anjaneyulu A S R, Rao G V. Chemical constituents of the soft coral species of Sarcophyton genus:a review[J]. J Indian Chem Soc, 1997, 74(4): 272–278. |
| [3] | Jiang C S, Müller W E G, Schröder H C, et al. Disulfide-and multisulfide-containing metabolites from marine organisms[J]. Chem Rev, 2012, 112(4): 2179–2207. DOI:10.1021/cr200173z |
| [4] | Liang L F, Wang X J, Zhang H Y, et al. Bioactive polyhydroxylated steroids from the Hainan soft coral Sinularia depressa Tixier-Durivault[J]. Bioorg Med Chem Lett, 2013, 23(5): 1334–1337. DOI:10.1016/j.bmcl.2012.12.087 |
| [5] | Liang L F, Wang T, Cai Y S, et al. Brominated polyunsaturated lipids from the Chinese sponge Xestospongia testudinaria as a new class of pancreatic lipase inhibitors[J]. Eur J Med Chem, 2014, 79C: 290–297. |
| [6] | Zhou Z F, Menna M, Cai Y S, et al. Polyacetylenes of marine origin:chemistry and bioactivity[J]. Chem Rev, 2015, 115(3): 1543–1596. DOI:10.1021/cr4006507 |
| [7] | Huang R Y, Chen W T, Kurtán T, et al. Bioactive isoquinolinequinone alkaloids from the South China Sea nudibranch Jorunna funebris and its sponge-prey Xestospongia sp[J]. Future Med Chem, 2016, 8(1): 17–27. DOI:10.4155/fmc.15.169 |
| [8] | Liang L F, Kurtán T, Mándi A, et al. Unprecedented diterpenoids as a PTP1B inhibitor from the Hainan soft coral Sarcophyton trocheliophorum Marenzeller[J]. Org Lett, 2013, 15(2): 274–277. DOI:10.1021/ol303110d |
| [9] | Liang L F, Gao L X, Li J, et al. Cembrane diterpenoids from the soft coral Sarcophyton trocheliophorum Marenzeller as a new class of PTP1B inhibitors[J]. Bioorg Med Chem, 2013, 21(17): 5076–5080. DOI:10.1016/j.bmc.2013.06.043 |
| [10] | Liang L F, Lan L F, Taglialatela-Scafati O, et al. Sartrolides A-G and bissartrolide, new cembranolides from the South China Sea soft coral Sarcophyton trocheliophorum Marenzeller[J]. Tetrahedron, 2013, 69(35): 7381–7386. DOI:10.1016/j.tet.2013.06.068 |
| [11] | Liang L F, Kurtán T, Mándi A, et al. Sarsolenane and capnosane diterpenes from the Hainan soft coral Sarcophyton trocheliophorum Marenzeller as PTP1B inhibitors[J]. Eur J Org Chem, 2014, 2014(9): 1841–1847. DOI:10.1002/ejoc.v2014.9 |
| [12] | Gutiérrez M, Pereira A R, Debonsi H M, et al. Cannabinomimetic lipid from a marine cyanobacterium[J]. J Nat Prod, 2011, 74(10): 2313–2317. DOI:10.1021/np200610t |
| [13] | 贾睿, 冯林音, 严小红, 等. 中国南海多棘软珊瑚的化学成分和生物活性研究[J]. 中国海洋药物, 2013, 32(1):13–16. |
| [14] | Bowden B F, Coll J C, Mitchell S J. Studies of Australian soft corals. XVⅢ. Further cembranoid diterpenes from soft corals of the genus Sarcophyton[J]. Aust J Chem, 1980, 33(4): 879–884. DOI:10.1071/CH9800879 |
| [15] | Jia R, Guo Y W, Mollo E, et al. Sarcophytonolides A-D, four new cembranolides from the Hainan soft coral Sarcophyton sp[J]. Helv Chim Acta, 2005, 88(5): 1028–1033. DOI:10.1002/(ISSN)1522-2675 |
| [16] | Toth J A, Burreson B J, Scheuer P J, et al. Emblide, a new polyfunctional cembranolide from the soft coral Sarcophyton glaucum[J]. Tetrahedron, 1980, 36(10): 1307–1309. DOI:10.1016/0040-4020(80)85041-1 |
| [17] | Gross H, Wright A D, Beil W, et al. Two new bicyclic cembranolides from a new Sarcophyton species and determination of the absolute configuration of sarcoglaucol-16-one[J]. Org Biomol Chem, 2004, 2(8): 1133–1138. DOI:10.1039/B314332E |
| [18] | Zhang C X, Li J, Su J Y, et al. Cytotoxic diterpenoids from the soft coral Sarcophyton crassocaule[J]. J Nat Prod, 2006, 69(10): 1476–1480. DOI:10.1021/np050499g |
| [19] | Dunlop R W, Wells R J. Isolation of some novel diterpenes from a soft coral of the genus Lobophytum[J]. Aust J Chem, 1979, 32(6): 1345–1351. DOI:10.1071/CH9791345 |
| [20] | 盛菲亚, 卢君蓉, 彭伟, 等. 香附炮制前后挥发油的GC-MS指纹图谱对比研究[J]. 中草药, 2013, 44(23):3321–3327. |
| [21] | Jiang C S, Liang L F, Guo Y W. Natural products possessing protein tyrosine phosphatase 1B (PTP1B) inhibitory activity found in the last decades[J]. Acta Pharmacol Sin, 2012, 33(10): 1217–1245. DOI:10.1038/aps.2012.90 |
 2016, Vol. 47
2016, Vol. 47


