甘草Glycyrrhizae RadixetRhizoma是豆科(Leguminosae)甘草属GlycyrrhizaLinn.某些植物的干燥根及根茎,广泛分布在我国的华北、东北和西北地区,素有“国老”的尊称[1]。《中国药典》2015年版中收录的甘草有3种,分别是甘草Glycyrrhiza uralensis Fisch.、胀果甘草Glycyrrhiza inflata Bat.和光果甘草Glycyrrhiza glabra L.。甘草具有补脾益气、清热解毒、祛痰止咳、缓急止痛、调和药效的功效[2],其主要的化学成分是黄酮类和三萜皂苷类等[3],还包括香豆素、生物碱、氨基酸、有机酸和多糖等。现代药理学研究表明,甘草具有抗肿瘤、抗动脉粥样硬化和抗血栓形成、抗病毒、抗炎、抗菌和抗原虫等药理作用[2-4]。目前已有研究证明甘草中的抗炎活性成分主要有甘草酸(glycyrrhizinic acid)、异甘草素(isoliquiritigenin)、异甘草苷(isoliquiritin)、甘草素(liquiritigenin)、甘草查耳酮A(licochalcone A)、光甘草定(glabridin)和甘草酚(glycyrol)等[4-10]。本实验对甘草乙醇提取液中的黄酮类化合物进行分离鉴定,共得到10个化合物,分别是甘草苷(liquiritin,1)、芹糖甘草苷(liquiritin apioside,2)、异甘草苷(isoliquiritin,3)、芹糖异甘草苷(isoliquiritin apioside,4)、sophoraisoflavone A(5)、粗毛甘草素F(glyasperin F,6)、光甘草酮(glabrone,7)、光甘草定(glabridin,8)、甘草黄酮醇(licoflavonol,9)和粗毛甘草素D(glyasperin D,10)。并用细菌脂多糖(LPS)诱导小鼠巨噬细胞RAW264.7炎症模型对分离到的化合物进行了抑制NO产生活性筛选,发现化合物1、3、5、6、8和9对NO分泌有一定的抑制作用。其中化合物5、6和9对抑制LPS诱导的RAW264.7细胞NO分泌的结果为首次报道,是甘草潜在的抗炎活性成分。
1 仪器与材料Agilent 1100型高效液相色谱仪(美国Agilent公司);Bruker Avance III 500核磁共振仪(瑞士Bruker公司);Agilent 6230 TOF质谱仪(美国Agilent公司);Agilent Zorbax SB-C18 Semi-Preparative色谱柱(250 mm×9.4 mm,5 μm,美国Agilent公司);Milli-Q水(法国Millipore公司);D101大孔树脂购自天津海光化工有限公司,ODS填料购自日本YMC公司,甲醇(分析纯)购自国药集团化学试剂有限公司,无水乙醇(分析纯)购自上海凌峰化学试剂有限公司;RAW264.7细胞购于上海细胞库,NO检测试剂盒购自上海碧云天生物技术有限公司,TECAN F200荧光及化学发光分析仪购自瑞士TCAN公司。
甘草(新疆,批号150501)购自杭州市本地药店,由浙江大学药学院陈柳蓉副教授鉴定为甘草Glycyrrhiza uralensis Fisch.的干燥根。
2 提取与分离取干燥的甘草5.0 kg,分别用8倍量和6倍量的95%乙醇水溶液回流提取2次,每次1 h。合并2次提取液,减压浓缩到一定浓度后,置于装有D101大孔树脂的色谱柱,分别用水和40%、95%乙醇洗脱,收集40%乙醇洗脱液,减压浓缩到适宜浓度后,进行半制备液相色谱分离,乙腈-水(10:90→100:0)梯度洗脱,得到化合物1(3.9 mg)、2(4.6 mg)、3(3.3 mg)和4(3.0 mg)。收集95%乙醇洗脱液,经ODS中压柱色谱进一步分离,使用55%甲醇和纯甲醇溶剂洗脱。收集纯甲醇洗脱液进行减压浓缩后冷冻干燥,得到干燥粉末(28 g)。取0.43 g干燥粉末用甲醇溶解,半制备液相色谱分离,乙腈-水(15:85→100:0)梯度洗脱,得到化合物5(2.8 mg)、6(10.8 mg)、7(1.2 mg)、8(3.0 mg)、9(2.7 mg)和10(2.1 mg)。
3 结构鉴定化合物1:白色粉末。ESI-MS m/z: 417 [M-H]−, 835 [2M-H]−。1H-NMR (500 MHz, CD3OD) δ: 5.46 (1H, dd, J=12.8, 3.0 Hz, H-2), 3.04 (1H, dd, J=16.9, 12.8 Hz, H-3a), 2.74 (1H, dd, J=16.9, 3.0 Hz, H-3b), 7.73 (1H, d, J=8.7 Hz, H-5), 6.51 (1H, dd, J=8.7, 2.3 Hz, H-6), 6.37 (1H, d, J=2.3 Hz, H-8), 7.44 (2H, d, J=8.6 Hz, H-2′, 6′), 7.15 (2H, d, J=8.7 Hz, H-3′, 5′), 4.94 (1H, d, J=7.5 Hz, H-1″), 3.90 (1H, dd, J=12.1, 2.2 Hz, H-6″a), 3.70 (1H, dd, J=12.1, 5.6 Hz, H-6″b), 3.37~3.49 (4H, m, H-2″~5″);13C-NMR (125 MHz, CD3OD) δ: 80.9 (C-2), 45.1 (C-3), 193.3 (C-4), 115.2 (C-4a), 134.6 (C-5), 112.0 (C-6), 167.0 (C-7), 104.0 (C-8), 165.6 (C-8a), 130.0 (C-1′), 128.9 (C-2′, 6′), 118.0 (C-3′, 5′), 159.4 (C-4′);glucose: 102.4 (C-1″), 75.1 (C-2″), 78.2 (C-3″), 71.5 (C-4″), 78.4 (C-5″), 62.7 (C-6″)。以上数据与文献报道[11]基本一致,故鉴定化合物1为甘草苷。
化合物2:白色粉末。ESI-MS m/z: 549 [M-H]−, 1 099 [2M-H]−。1H-NMR (500 MHz, CD3OD) δ: 5.45 (1H, dd, J=13.1, 3.1 Hz, H-2), 3.04 (1H, dd, J=16.9, 12.8 Hz, H-3a), 2.74 (1H, dd, J=17.0, 2.7 Hz, H-3b), 7.73 (1H, d, J=8.7 Hz, H-5), 6.51 (1H, dd, J=8.7, 2.3 Hz, H-6), 6.37 (1H, d, J=2.2 Hz, H-8), 7.44 (2H, d, J=8.4 Hz, H-2′, 6′), 7.12 (2H, d, J=8.7 Hz, H-3′, 5′), 5.00 (1H, d, J=7.5 Hz, H-1″), 3.89 (1H, dd, J=12.1, 2.2 Hz, H-6″a), 3.69 (1H, dd, J=12.0, 5.5 Hz, H-6″b), 3.47~3.67 (4H, m, H-2″~5″), 5.47 (1H, d, J=1.5 Hz, H-1′′′), 3.95 (1H, d, J=1.5 Hz, H-2′′′), 3.55 (2H, d, J=2.3 Hz, H-4′′′), 4.06 (1H, d, J=9.6 Hz, H-5′′′a), 3.80 (1H, d, J=9.5 Hz, H-5′′′b);13C-NMR (125 MHz, CD3OD) δ: 80.9 (C-2), 45.1 (C-3), 193.3 (C-4), 115.2 (C-4a), 130.0 (C-5), 112.0 (C-6), 166.9 (C-7), 104.0 (C-8), 165.6 (C-8a), 134.5 (C-1′), 129.0 (C-2′, 6′), 117.9 (C-3′, 5′), 159.2 (C-4′);glucose: 101.0 (C-1″), 78.2 (C-2″), 78.2 (C-3″), 71.6 (C-4″), 78.8 (C-5″), 62.7 (C-6″);apiose: 111.0 (C-1′′′), 78.8 (C-2′′′), 80.9 (C-3′′′), 75.6 (C-4′′′), 66.2 (C-5′′′)。以上数据与化合物1非常相似,仅比化合物1多出1组芹糖信号,参考文献报道[12],鉴定化合物2为芹糖甘草苷。
化合物3:黄色粉末。ESI-MS m/z: 417 [M-H]−, 835 [2M-H]−。1H-NMR (500 MHz, CD3OD) δ: 7.69 (1H, d, J=15.3 Hz, H-α), 7.81 (1H, d, J=15.3 Hz, H-β), 7.72 (2H, d, J=8.6 Hz, H-2, 6), 7.16 (2H, d, J=8.7 Hz, H-3, 5), 6.30 (1H, d, J=2.4 Hz, H-3′), 6.42 (1H, dd, J=8.9, 2.4 Hz, H-5′), 7.99 (1H, d, J=8.9 Hz, H-6′), 5.00 (1H, d, J=7.6 Hz, H-1″), 3.91 (1H, dd, J=12.2, 2.3 Hz, H-6″a), 3.71 (1H, dd, J=12.1, 5.7 Hz, H-6″b);3.38~3.51 (4H, m, H-2″~5″);13C-NMR (125 MHz, CD3OD) δ: 120.3 (C-α), 144.9 (C-β), 193.5 (C=O), 130.7 (C-1), 131.5 (C-2, 6), 118.2 (C-3, 5), 161.2 (C-4), 114.8 (C-1′), 166.8 (C-2′), 104.0 (C-3′), 167.7 (C-4′), 109.4 (C-5′), 133.6 (C-6′);glucose: 102.0 (C-1″), 75.0 (C-2″), 78.1 (C-3″), 71.5 (C-4″), 78.4 (C-5″), 62.7 (C-6″)。以上数据与文献报道[13]基本一致,故鉴定化合物3为异甘草苷。
化合物4:黄色粉末。ESI-MS m/z: 549 [M-H]−, 1 099 [2M-H]−。1H-NMR (500 MHz, CD3OD) δ: 7.70 (1H, d, J=15.1 Hz, H-α), 7.81 (1H, d, J=15.3 Hz, H-β), 7.72 (2H, d, J=8.7 Hz, H-2, 6), 7.14 (2H, d, J=8.8 Hz, H-3, 5), 6.30 (1H, d, J=2.4 Hz, H-3′), 6.43 (1H, dd, J=8.9, 2.4 Hz, H-5′), 7.99 (1H, d, J=8.9 Hz, H-6′), 5.06 (1H, d, J=7.5 Hz, H-1″), 3.91 (1H, dd, J=12.2, 2.3 Hz, H-6″a), 3.70 (1H, dd, J=12.2, 5.8 Hz, H-6″b);3.37~3.69 (4H, m, H-2″~5″), 5.47 (1H, d, J=1.6 Hz, H-1′′′), 3.95 (1H, d, J=1.6 Hz, H-2′′′), 3.54 (2H, d, J=1.4 Hz, H-4′′′), 4.06 (1H, d, J=9.6 Hz, H-5′′′a), 3.80 (1H, d, J=9.6 Hz, H-5′′′b);13C-NMR (125 MHz, CD3OD) δ: 120.3 (C-α), 145.0 (C-β), 193.6 (C=O), 130.7 (C-1), 131.6 (C-2, 6), 118.0 (C-3, 5), 161.1 (C-4), 114.9 (C-1′), 166.7 (C-2′), 104.0 (C-3′), 167.7 (C-4′), 111.0 (C-5′), 133.6 (C-6′);glucose: 100.7 (C-1″), 78.2 (C-2″), 78.3 (C-3″), 71.5 (C-4″), 78.8 (C-5″), 62.6 (C-6″);apiose: 109.2 (C-1′′′), 80.9 (C-2′′′), 78.7 (C-3′′′), 75.6 (C-4′′′), 66.2 (C-5′′′)。以上数据与化合物3非常相似,仅比化合物3多出1组芹糖信号,参考文献报道[14],鉴定化合物4为芹糖异甘草苷。
化合物5:淡黄色粉末。TOF-MS m/z: 351.090 8 [M-H]−。1H-NMR (500 MHz, CD3OD) δ: 7.93 (1H, s, H-2), 6.23 (1H, d, J=2.1 Hz, H-6), 6.35 (1H, d, J=2.1 Hz, H-8), 6.40 (1H, d, J=8.4 Hz, H-5′), 6.95 (1H, d, J=8.4 Hz, H-6′), 6.68 (1H, d, J=10.0 Hz, H-4″), 5.60 (1H, d, J=10.0 Hz, H-3″), 1.36 (6H, s, 2×CH3);13C-NMR (125 MHz, CD3OD) δ: 156.5 (C-2), 122.1 (C-3), 182.5 (C-4), 106.4 (C-4a), 163.9 (C-5), 100.3 (C-6), 166.1 (C-7), 95.0 (C-8), 159.9 (C-8a), 111.5 (C-1′), 153.3 (C-2′), 111.1 (C-3′), 155.1 (C-4′), 108.5 (C-5′), 132.4 (C-6′), 118.3 (C-4″), 129.7 (C-3″), 77.5 (C-2″), 28.2 (5″/6″-CH3), 27.9 (6″/5″-CH3)。以上数据与文献报道[15]基本一致,故鉴定化合物5为sophoraisoflavone A。
化合物6:黄色粉末。TOF-MS m/z: 353.101 7 [M-H]−。1H-NMR (500 MHz, CD3OD) δ: 4.58 (1H, dd, J=11.1, 10.0 Hz, H-2), 4.43 (1H, dd, J=11.0, 5.5 Hz, H-2), 4.19 (1H, dd, J=10.0, 5.5 Hz, H-3), 5.89 (1H, d, J=2.2 Hz, H-6), 5.89 (1H, d, J=2.2 Hz, H-8), 6.31 (1H, d, J=8.3 Hz, H-5′), 6.87 (1H, d, J=8.3 Hz, H-6′), 6.65 (1H, d, J=10.0 Hz, H-4″), 5.65 (1H, d, J=9.9 Hz, H-3″), 1.38 (6H, s, 2×CH3);13C-NMR (125 MHz, CD3OD) δ: 71.6 (C-2), 48.3 (C-3), 199.3 (C-4), 103.7 (C-4a), 166.0 (C-5), 97.3 (C-6), 168.5 (C-7), 96.2 (C-8), 165.3 (C-8a), 117.1 (C-1′), 152.3 (C-2′), 112.5 (C-3′), 154.9 (C-4′), 110.0 (C-5′), 130.8 (C-6′), 118.1 (C-4″), 130.6 (C-3″), 76.6 (C-2″), 28.1 (5″/6″-CH3), 28.0 (6″/5″-CH3)。以上数据与文献报道[16]基本一致,故鉴定化合物6为粗毛甘草素F。
化合物7:棕黄色粉末。TOF-MS m/z: 335.104 1 [M-H]−。1H-NMR (500 MHz, CD3OD) δ: 8.21 (1H, s, H-2), 8.11 (1H, d, J=8.9 Hz, H-5), 6.99 (1H, dd, J=8.5, 2.1 Hz, H-6), 6.90 (1H, d, J=2.2 Hz, H-8), 6.39 (1H, d, J=8.4 Hz, H-5′), 6.99 (1H, d, J=8.4 Hz, H-6′), 6.76 (1H, d, J=10.0 Hz, H-1″), 5.65 (1H, d, J=10.0 Hz, H-2″), 1.41 (6H, s, 2×CH3);13C-NMR (125 MHz, CD3OD) δ: 157.1 (C-2), 124.9 (C-3), 179.8 (C-4), 117.5 (C-4a), 128.8 (C-5), 117.3 (C-6), 165.6 (C-7), 103.3 (C-8), 160.0 (C-8a), 114.3 (C-1′), 153.3 (C-2′), 112.8 (C-3′), 156.1 (C-4′), 110.1 (C-5′), 131.5 (C-6′), 118.6 (C-4″), 130.2 (C-3″), 77.1 (C-2″), 28.2 (2×CH3)。以上数据与文献报道[17]基本一致,故鉴定化合物7为光甘草酮。
化合物8:黄色粉末。ESI-MS m/z: 323 [M-H]−。1H-NMR (500 MHz, CD3OD) δ: 4.30 (1H, ddd, J=10.2, 3.6, 2.1 Hz, H-2), 3.96 (1H, t, J=10.2 Hz, H-2), 3.42 (1H, m, H-3), 2.94 (1H, dd, J=15.6, 11.2 Hz, H-4), 2.76 (1H, ddd, J=15.6, 5.2, 2.0 Hz, H-4), 6.80 (1H, d, J=8.2 Hz, H-5), 6.27 (1H, d, J=8.3 Hz, H-6), 6.32 (1H, d, J=2.4 Hz, H-3′), 6.26 (1H, dd, J=8.3, 2.9 Hz, H-5′), 6.88 (1H, d, J=8.4 Hz, H-6′), 6.61 (1H, d, J=9.9 Hz, H-4″), 5.57 (1H, d, J=9.9 Hz, H-3″), 1.37 (6H, s, 2×CH3);13C-NMR (125 MHz, CD3OD) δ: 71.6 (C-2), 33.2 (C-3), 31.9 (C-4), 116.4 (C-4a), 131.6 (C-5), 109.6 (C-6), 153.2 (C-7), 111.1 (C-8), 151.2 (C-8a), 120.2 (C-1′), 157.4 (C-2′), 103.8 (C-3′), 158.2 (C-4′), 107.9 (C-5′), 129.9 (C-6′), 118.3 (C-4″), 130.4 (C-3″), 76.7 (C-2″), 28.1 (5″/6″-CH3), 28.0 (6″/5″-CH3)。以上数据与文献报道[18]基本一致,故鉴定化合物8为光甘草定。
化合物9:黄色粉末。TOF-MS m/z: 353.106 9 [M-H]−。1H-NMR (500 MHz, CD3OD) δ: 6.43 (1H, s, H-8), 8.08 (2H, d, J=8.4 Hz, H-2′, 6′), 6.90 (2H, d, J=8.5 Hz, H-3′, 5′), 3.33 (2H, m, H-1″, 与氘代试剂信号部分重合), 5.24 (1H, t, J=7.3 Hz, H-2″), 1.79 (3H, s, 4″-CH3), 1.67 (3H, s, 5″-CH3);13C-NMR (125 MHz, CD3OD) δ: 147.9 (C-2), 137.2 (C-3), 177.5 (C-4), 104.5 (C-4a), 160.6 (C-5), 112.4 (C-6), 163.6 (C-7), 93.8 (C-8), 156.4 (C-8a), 124.1 (C-1′), 130.8 (C-2′, 6′), 116.5 (C-3′, 5′), 159.3 (C-4′), 22.4 (C-1″), 123.7 (C-2″), 132.1 (C-3″), 26.1 (5″-CH3), 18.0 (4″-CH3)。以上数据与文献报道[15]基本一致,故鉴定化合物9为甘草黄酮醇。
化合物10:棕色粉末。TOF-MS m/z: 369.1805 [M-H]−。1H-NMR (500 MHz, CD3OD) δ: 4.21 (1H, ddd, J=10.3, 3.5, 1.9 Hz, H-2), 3.96 (1H, t, J=10.1 Hz, H-2), 3.35 (1H, m, H-3), 2.89 (1H, ddd, J=16.0, 5.5, 1.9 Hz, H-4), 2.80 (1H, dd, J=16.0, 10.7 Hz, H-4), 3.69 (3H, s, 5-OCH3), 3.74 (3H, s, 7-OCH3), 6.22 (1H, s, H-8), 6.32 (1H, d, J=2.5 Hz, H-3′), 6.27 (1H, dd, J=8.3, 2.5 Hz, H-5′), 6.89 (1H, d, J=8.3 Hz, H-6′), 3.24 (1H, m, H-1″), 5.14 (1H, t, J=7.1 Hz, H-2″), 1.75 (3H, s, 4″-CH3), 1.65 (3H, s, 5″-CH3);13C-NMR (125 MHz, CD3OD) δ: 71.2 (C-2), 32.9 (C-3), 26.9 (C-4), 109.7 (C-4a), 158.4 (C-5), 61.2 (5-OCH3), 116.4 (C-6), 158.7 (C-7), 56.2 (7-OCH3), 96.8 (C-8), 155.3 (C-8a), 120.4 (C-1′), 157.4 (C-2′), 103.8 (C-3′), 158.2 (C-4′), 107.8 (C-5′), 128.9 (C-6′), 23.6 (C-1″), 125.6 (C-2″), 131.1 (C-3″), 26.0 (5″-CH3), 18.0 (4″-CH3)。以上数据与文献报道[19]基本一致,故鉴定化合物10为粗毛甘草素D。
4 抗炎活性评价采用细菌脂多糖(LPS)诱导小鼠巨噬细胞RAW264.7炎症模型评价化合物抑制NO产生的活性。将RAW264.7细胞接种于96孔板,用MTT法测定各化合物的细胞毒性。确定无毒浓度后,以吲哚美辛(50.0 μmol/L)作阳性对照,用LPS(200 ng/mL)诱导小鼠巨噬细胞RAW264.7模型,以NO抑制率作抗炎活性指标,对各单体化合物进行抗炎活性初筛(n=3),对有抗炎活性的化合物做进一步的量效考察。
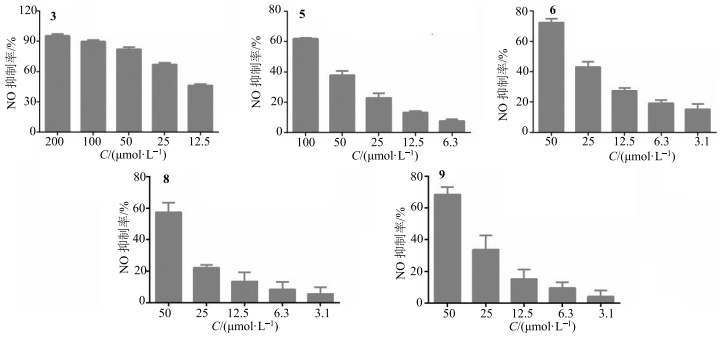
实验结果显示,异甘草苷(3)、sophoraisoflavone A(5)、粗毛甘草素F(6)、光甘草定(8)和甘草黄酮醇(9)有较强的抑制NO产生活性,而甘草苷(1)在50.0 μmol/L表现出较弱的NO抑制作用(抑制率为27%),芹糖甘草苷(2,200.0 μmol/L)、芹糖异甘草苷(4,200.0 μmol/L)、光甘草酮(7,50.0 μmol/L)和粗毛甘草素D(10,12.5 μmol/L)未显示有抑制活性。其中对甘草苷、芹糖甘草苷、异甘草苷和芹糖异甘草苷的实验结果分析可知,化合物结构中增加糖单元(芹糖),会降低对NO的抑制作用。对具有抑制NO产生活性的化合物3、5、6、8、9进行量效关系考察,根据毒性实验结果确定各化合物浓度梯度,结果见图 1,其半数抑制浓度(IC50)值依次为(14.0±1.8)、(70.3±4.0)、(26.5±2.0)、(22.4±2.0)、(17.0±2.1)μmol/L。与阳性对照吲哚美辛IC50(31.6±2.0)μmol/L[20]相比,化合物3、6、8、9有更好的抑制NO产生活性。
|
图 1 化合物3、6、8、9抑制NO产生活性的量效结果 Fig.1 Dose-effect result of inhibitory effect of compounds 3, 5, 6, 8, and 9 on NO production |
| [1] | 傅克治, 傅密宁. 甘草用途多[J]. 植物杂志 , 1987 (3) :12–13. |
| [2] | 杨莉, 陈海霞, 高文远, 等. 甘草抗肿瘤活性成分的研究[J]. 天然产物研究与开发 , 2009, 21 (3) :438–440. |
| [3] | Zhang Q Y, Ye M. Chemical analysis of the Chinese herbal medicine Gan-Cao (Licorice)[J]. J Chromatogr A , 2009, 1216 (11) :1954–1969. DOI:10.1016/j.chroma.2008.07.072 |
| [4] | 张明发, 沈雅琴. 甘草及其活性成分抗炎与抗炎机制的研究进展[J]. 现代药物与临床 , 2011, 26 (4) :261–268. |
| [5] | Fujisawa Y, Sakamoto M, Matsushita M, et al. Glycyrrhizin inhibits the lytic pathway of complement-possible mechanism of its anti-inflammatory effect on liver cells in viral hepatitis[J]. Microbiol Immunol , 2000, 44 (9) :799–804. DOI:10.1111/mim.2000.44.issue-9 |
| [6] | Kolbe L, Immeyer J, Batzer J, et al. Anti-inflammatory efficacy of licochalcone A:Correlation of clinical potency and in vitro effects[J]. Arch Dermatol Res , 2006, 298 (1) :23–30. DOI:10.1007/s00403-006-0654-4 |
| [7] | Peng F, Du Q H, Peng C, et al. A review:The pharmacology of isoliquiritigenin[J]. Phytother Res , 2015, 29 (7) :969–977. DOI:10.1002/ptr.v29.7 |
| [8] | Kim J Y, Park S J, Yun K J, et al. Isoliquiritigenin isolated from the roots of Glycyrrhiza uralensis inhibits LPS-induced NOS and COX-2 expression via the attenuation of NF-kappa B in RAW 264.7 macrophages[J]. Eur J Pharmacol , 2008, 584 (1) :175–184. DOI:10.1016/j.ejphar.2008.01.032 |
| [9] | Yu J Y, Ha J Y, Kim K M, et al. Anti-inflammatory activities of licorice extract and its active compounds, glycyrrhizic acid, liquiritin and liquiritigenin, in BV2 cells and mice liver[J]. Molecules , 2015, 20 (7) :13041–13054. DOI:10.3390/molecules200713041 |
| [10] | Shin E M, Zhou H Y, Guo L Y, et al. Anti-inflammatory effects of glycyrol isolated from Glycyrrhiza uralensis in LPS-stimulated RAW264. 7 macrophages[J]. Int Immunopharmacol , 2008, 8 (11) :1524–1532. DOI:10.1016/j.intimp.2008.06.008 |
| [11] | 蒋晓文, 白俊鹏, 田星, 等. 牛蒡根中黄酮苷类化学成分及其抗氧化活性构效关系的研究[J]. 中草药 , 2016, 47 (5) :726–731. |
| [12] | Xu J, Luo J, Kong L. Simultaneous separation of triterpenoid saponins and flavonoid glycosides from the roots of Glycyrrhiza uralensis Fisch. by pH-zone-refining counter-current chromatography[J]. J Sep Sci , 2013, 36 (19) :3295–3301. |
| [13] | Fu B, Li H, Wang X, et al. Isolation and identification of flavonoids in licorice and a study of their inhibitory effects on tyrosinase[J]. J Agric Food Chem , 2005, 53 (19) :7408–7414. DOI:10.1021/jf051258h |
| [14] | Kitagawa I, Hori K, Uchida E, et al. Saponin and sapogenol L. on the constituents of the roots of Glycyrrhiza uralensis Fischer from Xinjiang, China. Chemical structures of licorice-saponin L3 and isoliquiritin apioside[J]. Chem Pharm Bull , 1993, 41 (9) :1567–1572. DOI:10.1248/cpb.41.1567 |
| [15] | Shirataki Y, Yokoe I, Noguchi M, et al. Studies on the constituents of Sophora species XXⅡ. Constituents of the root of Sophora moorcroftiana Benth ex Baker[J]. Chem Pharm Bull , 1988, 36 (6) :2220–2225. DOI:10.1248/cpb.36.2220 |
| [16] | Mckee T C, Bokesch H R, Mccormick J L, et al. Isolation and characterization of new anti-HIV and cytotoxic leads from plants, marine, and microbial organisms[J]. J Nat Prod , 1997, 60 (5) :431–438. DOI:10.1021/np970031g |
| [17] | Kinoshita T, Tamura Y, Mizutani K. Isolation and synthesis of two new 3-arylcoumarin derivatives from the root of Glycyrrhiza glabra (Licorice), and structure revision of an antioxidant isoflavonoid glabrene[J]. Nat Prod Lett , 1997, 9 (4) :289–296. DOI:10.1080/10575639708043642 |
| [18] | Jirawattanapong W, Saifah E, Patarapanich C. Synthesis of glabridin derivatives as tyrosinase inhibitors[J]. Arch Pharm Res , 2009, 32 (5) :647–654. DOI:10.1007/s12272-009-1501-x |
| [19] | 王青, 苗文娟, 向诚, 等. 乌拉尔甘草中黄酮类化学成分的研究[J]. 中草药 , 2014, 45 (1) :31–36. |
| [20] | Wang S F, Xu Y M, Jiang W, et al. Isolation and identification of constituents with activity of inhibiting nitric oxide production in Raw 264.7 macrophages from Gentiana triflora[J]. Planta Med , 2013, 79 (8) :680–686. DOI:10.1055/s-00000058 |
 2016, Vol. 47
2016, Vol. 47


