2. 天津中医药大学中医药研究院, 天津 300193 ;
3. 天津市现代中药重点实验室, 天津 300193
2. Institute of Traditional Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin 300193, China ;
3. Tianjin State Key Laboratory of Modern Chinese Medicine, Tianjin 300193, China
黄柏Phellodendri Cortex为芸香科(Rutaceae)植物黄皮树Phellodendron chinense Schneid. 的干燥树皮,习称“川黄柏”,是临床常用中药。黄柏味苦,性寒,归肾、膀胱经,具有清热燥湿、泻火除蒸、解毒疗疮的功效,用于治疗湿热泻痢、黄疸尿赤、带下阴痒、热淋涩痛、脚气痿躄、骨蒸劳热、盗汗、遗精、疮疡肿毒、湿疹湿疮[1]。黄柏的化学成分报道最早见于日本学者村山义温等对日本产黄檗Phellodendron amurense Rupr. 的研究,从中分离得到小檗碱(berberine)及少量巴马汀(palmatine),此后各国学者陆续报道了其他的化学成分[2]。至今,对黄柏的化学成分和药理作用的研究大部分集中在生物碱部分,对非生物碱部分的研究相对较少,因此,为了深入研究黄柏药理活性的物质基础,本课题对黄柏70%乙醇提取物经阳离子交换树脂处理后得到的非生物碱部分进行较深入的化学成分研究,从中分离得到14个化合物,分别鉴定为 (−)-3-O-阿魏酰奎宁酸甲酯 [(−)-3-O-feruloylquinic acid methyl ester,1]、(−)-4-O-阿魏酰奎宁酸甲酯 [(−)-4-O-feruloylquinic acid methyl ester,2]、(−)-绿原酸甲酯 [(−)-chlorogenic acid methyl ester,3]、(−)-5-O-阿魏酰奎宁酸甲酯 [(−)-5-O-feruloylquinic acid methyl ester,4]、毛柳苷(salidroside,5)、阿魏酸甲酯(methyl ferulate,6)、咖啡酸甲酯(caffeic acid methyl ester,7)、4-羟基苯乙醇(p-hydroxyl- benzyl ethanol,8)、3,4,5-三甲氧基苯酚-O-β-D-葡萄糖苷(3,4,5-trimetoxy phenol-O-β-D-glucopyranoside,9)、2-methoxy-4-(2-propenyl) phenyl-β-D-glucopyra- noside(10)、tachinoside(11)、丁香酸甲酯(methyl syringate,12)、(6S)-去氢催吐萝芙木 [(6S)-dehydro vomifoliol,13]、(6R,7aR)-地芰普内酯 [(6R,7aR)- epiloliolide,14]。其中化合物9~14均为首次从该属植物中分离得到。
1 仪器与材料Waters-e2695高效液相色谱仪;Waters-2535制备液相色谱仪;岛津LC-6AD型制备液相色谱仪;Bruker PLUS 400 MHz核磁共振波谱仪;Waters Xevo G2 Q-TOF MS质谱仪;AUTOPOL V旋光仪;柱层层析硅胶100~200、200~300目(青岛鼎康硅胶有限公司);ODS-A-HG 12 nm S-50 μm(日本YMC公司);Sephadex LH-20(Pharmacia,瑞典);D101大孔吸附树脂(天津波鸿树脂科技有限公司);732型强酸性阳离子交换树脂(天津市申泰化学试剂有限公司);所用试剂均为分析纯或者色谱纯(天津康科德试剂公司)。
黄柏药材经天津中医药大学张丽娟教授鉴定为黄皮树Phellodendron chinense Schneid. 的干燥树皮,现保存于天津中医药大学中药学院。
2 提取与分离黄柏干燥树皮9.0 kg,70%乙醇-水10倍量回流提取3次,每次2 h,合并提取液,48 ℃条件下减压回收溶剂,浓缩后得到总浸膏,用水(5 L)分散,4%稀硫酸调pH值为4~5后,经732型阳离子交换树脂富集生物碱,得到非生物碱435 g(经TLC检测,碘化铋钾反应呈阴性)。
非生物碱经D101大孔吸附树脂以乙醇-水(0∶100→95∶5)梯度初步洗脱,得到5个粗流分(Fr. 1~5)。因各流分间存在部分交叉,故将Fr. 2(100 g)再次经D101大孔吸附树脂以乙醇-水(10∶90→95∶5)梯度洗脱,得到3个流分(Fr. 2-1~2 -3);将Fr. 3(50 g)再次经D101大孔吸附树脂以乙醇-水(10∶90→95∶5)梯度洗脱,得到8个流分(Fr. 3-1~3-8)。
Fr. 2-1(28 g)经ODS柱色谱洗脱,Sephadex LH-20凝胶柱色谱洗脱以及制备HPLC(甲醇-水30∶70)得到化合物1(12 mg)和5(33.6 mg)。Fr. 2-2(4 g)经Sephadex LH-20凝胶柱洗脱以及制备HPLC(甲醇-水35∶65)纯化得到化合物2(20 mg)、3(154 mg)、4(135 mg)。
Fr. 3-1经ODS柱色谱洗脱,Sephadex LH-20凝胶柱色谱洗脱,得到化合物7(13.4 mg)。Fr. 3-2经Sephadex LH-20柱色谱洗脱以及制备HPLC(甲醇-水45∶55)纯化得到化合物8(21.7 mg)和9(9.5 mg)。Fr. 3-4(145 mg)经Sephadex LH-20柱色谱洗脱以及制备HPLC(甲醇-水35∶65)纯化得到化合物10(38.4 mg)和11(23.4 mg)。Fr. 3-5(130 mg)经Sephadex LH-20柱色谱洗脱以及制备HPLC(甲醇-水35∶65)得到化合物12(41.4 mg)。Fr. 3-6(180 mg)经硅胶柱色谱和Sephadex LH-20柱色谱反复纯化得到化合物13(11.2 mg)和14(14.9 mg)。Fr. 3-7(2 g)经硅胶柱色谱,CH2Cl2-MeOH(100∶0→0∶100)梯度洗脱和Sephadex LH-20柱色谱洗脱得到化合物6(22.7 mg)。
3 结构鉴定化合物1:白色粉末(CH3OH);[α]D25 −28.16°(MeOH,c 0.12);HR-ESI-MS m/z: 381.121 4 [M-H]−(计算值382.126 4),结合1H-NMR和13C-NMR数据确定分子式为C18H22O9。1H-NMR (400 MHz,DMSO-d6) δ: 7.56 (1H,d,J = 15.6 Hz,H-7),7.29 (1H,d,J = 1.6 Hz,H-2),7.10 (1H,dd,J = 8.0,1.6 Hz,H-6),6.78 (1H,d,J = 8.0 Hz,H-5),6.45 (1H,d,J = 15.6 Hz,H-8),5.17 (1H,m,H-3′),4.00 (1H,m,H-4′),3.82 (3 H,s,3-OCH3),3.61 (1H,m,H-5′),3.59 (3H,s,-COOCH3),2.05 (2H,m,H-6′),1.90 (2H,m,H-2′);13C-NMR (100 MHz,DMSO-d6) δ: 174.2 (C=O),166.1 (C-9),149.1 (C-4),148.0 (C-3),144.5 (C-7),125.7 (C-1),123.0 (C-6),115.5 (C-5,8),111.0 (C-2),72.6 (C-1′),70.3 (C-3′),69.7 (C-4′),67.8 (C-5′),55.7 (3-OCH3),51.5 (COOCH3),38.1 (C-6′),35.0 (C-2′)。以上数据与文献报道基本一致[3],故鉴定化合物1为 (−)-3-O-阿魏酰奎宁酸甲酯。
化合物2:黄色油状物(CH3OH);[α]D25 −49.4° (MeOH,c 0.10);HR-ESI-MS m/z: 381.122 1 [M-H]−(计算值382.126 4),结合1H-NMR和13C-NMR数据确定分子式为C18H22O9。1H-NMR (400 MHz,CD3OD) δ: 7.63 (1H,d,J = 16.0 Hz,H-7),7.13 (1H,d,J = 1.9 Hz,H-2),7.02 (1H,dd,J = 8.0,1.9 Hz,H-6),6.75 (1H,d,J = 8.0 Hz,H-5),6.38 (1H,d,J = 16.0 Hz,H-8),4.75 (1H,dd,J = 3.0,2.5 Hz,H-4′),4.22 (1H,m,H-5′),4.16 (1H,m,H-3′),3.83 (3H,s,3-OCH3),3.68 (3H,s,-COOCH3),2.16 (2H,m,H-2′),1.93 (2H,m,H-6′);13C-NMR (100 MHz,CD3OD) δ: 175.8 (C=O),169.0 (C-9),150.8 (C-4),149.5 (C-3),147.1 (C-7),128.0 (C-1),124.2 (C-6),116.6 (C-5),115.9 (C-8),111.9 (C-2),78.7 (C-1′),76.5 (C-4′),69.1 (C-5′),65.9 (C-3′),56.6 (3-OCH3),53.1 (COOCH3),42.3 (C-6′),38.6 (C-2′)。以上数据与文献报道基本一致[4],故鉴定化合物2为 (−)-4-O-阿魏酰奎宁酸甲酯。
化合物3:绿色油状物(CH3OH;[α]D25 −108.1°(MeOH,c 0.08);HR-ESI-MS m/z: 367.105 7 [M-H]−(计算值368.110 7),结合1H-NMR和13C-NMR数据确定分子式为C17H20O9。1H-NMR (400 MHz,CD3OD) δ: 7.52 (1H,d,J = 16.0 Hz,H-7),7.03 (1H,d,J =1.9 Hz,H-2),6.94 (1H,dd,J = 8.0,1.9 Hz,H-6),6.78 (1H,d,J = 8.0 Hz,H-5),6.21 (1H,d,J = 16.0 Hz,H-8),5.27 (1H,m,H-5′),4.13 (1H,m,H-3′),3.73 (1H,m,H-4′),3.69 (3H,s,-COOCH3),2.18 (2H,m,H-6′),1.99~2.19 (2H,m,H-2′);13C-NMR (100 MHz,CD3OD) δ: 175.6 (C=O),168.4 (C-9),149.8 (C-4),147.3 (C-7),147.0 (C-3),127.8 (C-1),123.1 (C-6),116.7 (C-5),115.2 (C-2,8),76.0 (C-1′),72.7 (C-4′),72.2 (C-5′),70.5 (C-3′),53.1 (COOCH3),38.2 (C-2′),37.9 (C-6′)。以上数据与文献报道基本一致[5],故鉴定化合物3为 (−)-绿原酸甲酯。
化合物4:黄色油状物(CH3OH);[α]D25 −30.6°(MeOH,c 0.10);HR-ESI-MS m/z: 381.121 8 [M-H]−(计算值382.126 4),结合1H-NMR和13C-NMR数据确定分子式为C18H22O9。1H-NMR (400 MHz,CD3OD) δ: 7.51 (1H,d,J = 16.0 Hz,H-7),7.10 (1H,d,J = 1.6 Hz,H-2),7.00 (1H,dd,J = 8.0,1.6 Hz,H-6),6.74 (1H,d,J = 8.0 Hz,H-5),6.24 (1H,d,J = 16.0 Hz,H-8),5.21 (1H,m,H-5′),4.01 (1H,m,H-3′),3.82 (3H,s,3-OCH3),3.66 (1H,m,H-4′),3.62 (3H,s,-COOCH3),2.13 (2H,m,H-2′),2.02 (2H,m,H-6′);13C-NMR (100 MHz,CD3OD) δ: 175.6 (C=O),168.4 (C-9),151.0 (C-4),149.6 (C-3),147.2 (C-7),127.8 (C-1),124.3 (C-6),116.7 (C-5),115.6 (C-8),111.8 (C-2),76.0 (C-1′),72.8 (C-4′),72.3 (C-3′),70.5 (C-5′),56.6 (3-OCH3),53.1 (COOCH3),38.2 (C-2′,6′)。以上数据与文献报道基本一致[6],故鉴定化合物4为 (−)-5-O-阿魏酰奎宁酸甲酯。
化合物5:黄色油状物(MeOH);HR-ESI-MS m/z: 345.118 8 [M+COOH]−(计算值300.120 9),结合1H-NMR和13C-NMR数据确定分子式为C14H20O7。1H-NMR (400 MHz,DMSO-d6) δ: 9.17 (1H,s,-OH),7.03 (2H,d,J = 8.6 Hz,H-10,14),6.65 (2H,d,J = 8.6 Hz,H-11,13),4.96 (1H,d,J = 5.1 Hz,-OH),4.92 (1H,d,J = 5.1 Hz,-OH),4.88 (1H,d,J = 5.1 Hz,-OH),4.48 (1H,t,J = 5.8 Hz,-OH),4.15 (1H,d,J = 7.9 Hz,H-1),3.86 (1H,dt,J = 9.0,7.0 Hz,H-7a),3.65 (1H,ddd,J =11.7,6.1,1.8 Hz,H-6a),3.55 (1H,dt,J = 9.1,6.7 Hz,H-7b),3.42 (1H,dt,J = 11.7,5.9 Hz,H-6b),3.10 (1H,m,H-3),3.06 (1H,m,H-5),3.03 (1H,m,H-4),2.94 (1H,m,H-2),2.73 (2H,m,H-8);13C-NMR (100 MHz,DMSO-d6) δ: 155.6 (C-12),129.7 (C-10,14),128.6 (C-9),115.0 (C-11,13),102.8 (C-1),76.9 (C-5),76.8 (C-3),73.4 (C-2),70.1 (C-4),69.9 (C-7),61.1 (C-6),34.8 (C-8)。以上数据与文献报道基本一致[7],故鉴定化合物5为毛柳苷。
化合物6:白色粉末(MeOH);HR-ESI-MS m/z: 207.065 7 [M-H]−(计算值208.073 6),结合1H-NMR和13C-NMR数据确定分子式为C11H12O4。1H-NMR (400 MHz,CD3OD) δ: 7.61 (1H,d,J = 15.9 Hz,H-1′),7.18 (1H,d,J = 2.0 Hz,H-2),7.06 (1H,dd,J = 8.1,2.0 Hz,H-6),6.80 (1H,d,J = 8.1 Hz,H-5),6.35 (1H,d,J = 15.9 Hz,H-2′),3.89 (3H,s,3-OCH3),3.76 (3H,s,3′-OCH3);13C-NMR (100 MHz,CD3OD) δ: 169.9 (C-3′),150.8 (C-4),149.5 (C-3),147.0 (C-1′),127.8 (C-1),124.2 (C-6),116.6 (C-5),115.3 (C-2′),111.8 (C-2),56.6 (3-OCH3),52.1 (3′-OCH3)。以上数据与文献报道基本一致[8],故鉴定化合物6为阿魏酸甲酯。
化合物7:黄色粉末(MeOH);HR-ESI-MS m/z: 193.053 3 [M-H]−(计算值194.057 9),结合1H-NMR和13C-NMR数据确定分子式为C10H10O4。1H-NMR (400 MHz,CD3OD) δ: 7.53 (1H,d,J = 15.9 Hz,H-7),7.02 (1H,d,J = 2.0 Hz,H-2),6.93 (1H,dd,J = 8.1,2.0 Hz,H-6),6.77 (1H,d,J = 8.1 Hz,H-5),6.24 (1H,d,J = 16.0 Hz,H-8),3.75 (3H,s,3-OCH3);13C-NMR (100 MHz,CD3OD) δ: 169.9 (C-9),149.7 (C-4),147.1 (C-3),146.9 (C-7),127.9 (C-1),123.0 (C-6),116.6 (C-5),115.3 (C-8),115.0 (C-2),52.1 (3-OCH3)。以上数据与文献报道基本一致[9],故鉴定化合物7为咖啡酸甲酯。
化合物8:黄色固体(MeOH);HR-ESI-MS m/z: 137.023 5 [M-H]−(计算值138.068 1),结合1H-NMR和13C-NMR数据确定分子式为C8H10O2。1H-NMR (400 MHz,CD3OD) δ: 7.03 (2H,d,J = 8.2 Hz,H-2,6),6.70 (2H,d,J = 8.2 Hz,H-3,5),3.68 (2H,t,J = 7.2 Hz,H-8),2.71 (2H,t,J = 7.2 Hz,H-7);13C-NMR (100 MHz,CD3OD) δ: 156.9 (C-4),131.2 (C-1),131.1 (C-2,6),116.3 (C-3,5),64.7 (C-8),39.6 (C-7)。以上数据与文献报道基本一致[10],故鉴定化合物8为4-羟基苯乙醇。
化合物9:白色粉末(MeOH);HR-ESI-MS m/z: 391.124 7 [M+COOH]−(计算值346.126 4),结合1H-NMR和13C-NMR数据确定分子式为C15H22O9。1H-NMR (400 MHz,CD3OD) δ: 6.38 (2H,s,H-2,6),4.77 (1H,d,J = 5.0 Hz,H-1′),4.09 (2H,q,J = 5.3 Hz,H-6′),3.73 (6H,s,3,5-OCH3),3.58 (3H,s,4-OCH3),3.42 (1H,m,H-2′),3.26 (1H,m,H-5′),3.20 (1H,m,H-4′),3.10 (1H,m,H-3′);13C-NMR (100 MHz,CD3OD) δ: 154.0 (C-1),153.1 (C-3,5),132.4 (C-4),101.0 (C-1′),94.3 (C-2,6),77.3 (C-5′),76.8 (C-3′),73.2 (C-2′),70.1 (C-4′),60.9 (C-6′),60.1 (4-OCH3),55.7 (3,5-OCH3)。以上数据与文献报道基本一致[11],故鉴定化合物9为3,4,5-三甲氧基苯酚-O-β-D-葡萄糖苷。
化合物10:黄色油状物(MeOH);HR-ESI-MS m/z: 371.133 9 [M+COOH]−(计算值326.136 6),结合1H-NMR和13C-NMR数据确定分子式为C16H22O7。1H-NMR (400 MHz,CD3OD) δ: 7.08 (1H,d,J = 8.3 Hz,H-6),6.83 (1H,s,H-3),6.72 (1H,d,J = 8.3 Hz,H-5),5.94 (1H,m,H-8),5.05 (1H,d,J = 16.4 Hz,H-9′),5.02 (1H,d,J = 16.4 Hz,H-9′),4.84 (1H,d,J = 7.6 Hz,H-1′),3.86 (2H,q,J = 12.4,2.0 Hz,H-6′),3.85 (3H,s,H-10),3.42 (1H,m,H-2′),3.26 (1H,m,H-5′),3.20 (1H,m,H-4′),3.10 (1H,m,H-3′);13C-NMR (100 MHz,CD3OD) δ: 150.9 (C-2),146.5 (C-1),139.1 (C-8),136.6 (C-4),122.2 (C-5),118.4 (C-6),116.0 (2-OCH3),114.3 (C-3),103.2 (C-1′),78.3 (C-3′),78.0 (C-5′),75.1 (C-2′),71.5 (C-4′),62.6 (C-6′),56.8 (C-10),40.9 (C-7)。以上数据与文献报道基本一致[12],故鉴定化合物10为2-methoxy-4-(2- propenyl) phenyl-β-D-glucopyranoside。
化合物11:无色油状物(MeOH);HR-ESI-MS m/z: 347.097 5 [M+COOH]−(计算值302.100 2),结合1H-NMR和13C-NMR数据确定分子式为C13H18O8。1H-NMR (400 MHz,CD3OD) δ: 6.80 (1H,d,J = 2.2 Hz,H-3),6.69 (1H,d,J = 8.6 Hz,H-5),6.58 (1H,dd,J = 8.6,2.2 Hz,H-6),4.74 (1H,d,J = 7.2 Hz,H-1′),3.90 (1H,d,J = 12.0 Hz,H-6′a),3.85 (3H,s,2-OCH3),3.69 (1H,d,J = 5.1 Hz,H-6′b),3.66 (1H,m,H-3′),3.43 (1H,m,H-5′),3.38 (1H,m,H-2′),3.35 (1H,m,H-4′);13C-NMR (100 MHz,CD3OD) δ: 153.0 (C-4),149.4 (C-2),143.1 (C-1),116.1 (C-6),110.2 (C-5),104.0 (C-3),103.9 (C-1′),78.3 (C-3′),78.2 (C-5′),75.1 (C-2′),71.7 (C-4′),62.8 (C-6′),56.5 (2-OCH3)。以上数据与文献报道基本一致[13],故鉴定化合物11为tachinoside。
化合物12:黄色粉末(MeOH);HR-ESI-MS m/z: 211.061 1 [M-H]−(计算值212.068 5),结合1H-NMR和13C-NMR数据确定分子式为C10H12O5。1H-NMR (400 MHz,CD3OD) δ: 7.31 (2H,s),4.84 (6H,s),3.87 (3H,s);13C-NMR (100 MHz,CD3OD) δ: 168.8 (C-7),149.1 (C-3,5),142.0 (C-4),121.5 (C-1),108.3 (C-2,6),56.9 (3,5-OCH3),52.6 (7-OCH3)。以上数据与文献报道基本一致[14],故鉴定化合物12为丁香酸甲酯。
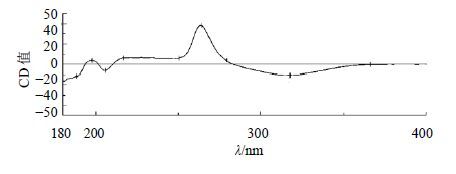
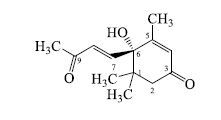
化合物13:黄色油状物(MeOH);[α]D25 +95.4°(MeOH,c 0.08);HR-ESI-MS m/z: 223.139 8 [M+H]+(计算值222.125 6),结合1H-NMR和13C-NMR数据确定分子式为C13H18O3。1H-NMR (400 MHz,CD3OD) δ: 7.01 (1H,d,J = 15.6 Hz,H-7),6.44 (1H,d,J = 15.6 Hz,H-8),5.93 (1H,s,H-4),2.62 (1H,d,J = 16.8 Hz,H-2a),2.31 (1H,d,J = 16.8 Hz,H-2b),2.31 (3H,s,H-10),1.90 (3H,s,H-13),1.06 (3H,s,H-11),1.02 (3H,s,H-12);13C-NMR (100 MHz,CD3OD) δ: 200.8 (C-9),200.5 (C-3),164.8 (C-5),148.5 (C-7),131.9 (C-8),128.2 (C-4),80.1 (C-6),50.7 (C-2),42.8 (C-1),27.8 (C-10),24.9 (C-12),23.7 (C-11),19.3 (C-13)。以上数据与文献报道基本一致[15-16],故鉴定化合物13为去氢催吐萝芙木醇。结构中6位为手性碳,CD谱中(图 1),在264 nm处显示正的Cotton效应 (Δε +38.4),结合文献报道[17],化合物 (+)-去氢催吐萝芙木醇在261 nm处有正的Cotton效应 (Δε +34.5),确定其构型为S,故最终确定化合物13为 (6S)-去氢催吐萝芙木醇。其结构见图 2。
|
图 1 化合物13的CD图谱 Fig.1 CD spectrum of compound 13 |
|
图 2 化合物13的结构式 Fig.2 Structure of compound 13 |
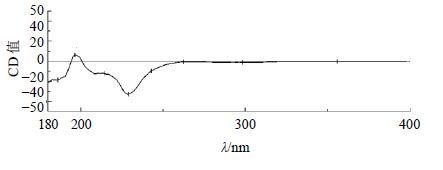
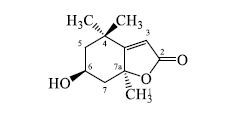
化合物14:白色粉末(MeOH);[α]D25 −88.9°(MeOH,c 0.09);HR-ESI-MS m/z: 197.1179 [M+H]+(计算值196.109 9),结合1H-NMR和13C-NMR数据确定分子式为C11H16O3。1H-NMR (400 MHz,CD3OD) δ: 5.75 (1H,s,H-3),4.22 (1H,q,J = 3.4 Hz,H-6),2.42 (1H,dt,J = 13.7,2.4 Hz,H-7a),2.00 (1H,dt,J = 14.3,2.5 Hz,H-5b),1.76 (3H,s,H-11),1.75 (1H,dd,J = 13.7,4.2 Hz,H-7b),1.53 (1H,dd,J = 14.3,3.8 Hz,H-5a),1.46 (3H,s,H-9),1.27 (3H,s,H-10);13C-NMR (100 MHz,CD3OD) δ: 185.9 (C-2),174.6 (C-8),113.5 (C-3),89.1 (C-7a),67.4 (C-6),48.1 (C-5),46.6 (C-7),37.3 (C-4),31.2 (C-10),27.6 (C-11),27.1 (C-9)。以上数据与文献报道基本一致[18],故鉴定化合物14为地芰普内酯。结构中6位和7a位为手性碳,CD谱中(图 3),在229 nm处显示负的Cotton效应 (Δε −32.9),结合文献报道[19],化合物 (6R,7aR)-地芰普内酯在223 nm处有负的Cotton效应,确定其构型为6R,7aR。故最终确定化合物14为 (6R,7aR)-地芰普内酯。其结构见图 4。
|
图 3 化合物14的CD图谱 Fig.3 CD spectrum of compound 14 |
|
图 4 化合物14的结构式 Fig.4 Structure of compound 14 |
4 讨论
采用各种柱色谱方法对黄柏乙醇提取物中的非生物碱部分进行分离纯化,并根据化合物的理化性质、波谱及光谱数据进行结构鉴定,共鉴定得到14个化合物,其中,3,4,5-三甲氧基苯酚-O-β-D-葡萄糖苷(9)、2-methoxy-4-(2-propenyl) phenyl-β-D- glucopyranoside(10)、tachinoside(11)、丁香酸甲酯(12)、(6S)-去氢催吐萝芙木醇(13)、(6R,7aR)-地芰普内酯(14)为首次从该属植物中分离得到,丰富了黄柏化学组分信息库,并为黄柏的药效研究提供更多的物质基础。
| [1] | 中国药典[S]. 一部. 2015. |
| [2] | 刘寿山. 中药研究文献摘要[M]. 北京: 科学出版社, 1963 . |
| [3] | 李云志, 马超, 黄静. 刮筋板的化学成分和抗肿瘤活性研究[J]. 中国药学杂志 , 2009, 44 (17) :1294–1297. |
| [4] | 董二会, 王爱国, 杨建波, 等. 川黄柏抗糖尿病活性部位化学成分研究[J]. 中药材 , 2012, 35 (9) :1441–1443. |
| [5] | Zhang M, Liu W X, Zheng M F, et al. Bioactive quinic acid derivatives from Ageratin aadenophora[J]. Molecules , 2013, 18 (11) :14096–14104. |
| [6] | 王珏, 王乃利, 姚新生, 等. 小花鬼针草中咖啡酰奎宁酸类成分及其抑制组胺释放活性[J]. 中草药 , 2006, 37 (7) :966–970. |
| [7] | Yu P Z, Hu C Q, Meehan E J, et al. X-Ray crystal structure and antioxidant activity of salidroside, a phenylethanoid glycoside[J]. Chem Biodivers , 2007, 4 (3) :508–513. |
| [8] | Subramaniam G, Subbarao G V, Kazuhiko N, et al. Nitrification inhibitors from the root tissues of Brachiaria humidicola, a tropical grass[J]. J Agric Food Chem , 2007, 55 (4) :1385–1388. |
| [9] | 赵晓宏, 陈迪华, 斯建勇, 等. 中药升麻酚酸类化学成分研究[J]. 药学学报 , 2002, 37 (7) :535–538. |
| [10] | 常东东, 戴好富, 左文健, 等. 海南龙血树内生真菌A12中的抗菌活性成分研究[J]. 中国抗生素杂志 , 2012, 37 (6) :421–424. |
| [11] | Saijo R, Nonaka G I, Nishioka I. Phenolic glucosides gallates from Mallotus japonicus[J]. Phytochemistry , 1989, 28 (9) :2443–2446. |
| [12] | Shimoda K, Kondo Y, Nishida T, et al. Biotransformation of thymol, carvacrol, and eugenol by cultured cells of Eucalyptus perriniana[J]. Phytochemistry , 2006, 67 (20) :2256–2261. |
| [13] | 冯卫生, 王建超, 何玉环, 等. 辛夷化学成分的研究[J]. 中国药学杂志 , 2015, 50 (24) :2103–2106. |
| [14] | 郑楠楠, 杨胜祥, 周慧, 等. 簕欓花椒的化学成分及生物活性研究[J]. 中草药 , 2015, 46 (2) :189–193. |
| [15] | Hisahiro K, Masaki B, Toru O. Two New megastigmanes from the leaves of Cucumis sativus[J]. Chem Pharm Bull , 2007, 55 (1) :133–136. |
| [16] | 朱玲娟, 燕菲, 陈金鹏, 等. 荚果蕨地上部分萜类化学成分研究[J]. 中草药 , 2015, 46 (12) :1737–1741. |
| [17] | Koreeda M, Weiss G, Nakanishi K. Absolute configuration of natural (+)-abscisic acid[J]. J Am Chem Soc , 1973, 95 (1) :239–240. |
| [18] | Marino S D, Borbone N, Gala F, et al. New constituents of sweet Capsicum annuum L. fruits and evaluation of their biological activity[J]. J Agric Food Chem , 2006, 54 (20) :7508–7516. |
| [19] | Schühly W, Fabian C, Jahangir S, et al. Structure and absolute configuration of loliolide isomers determined by comparison of calculated and experimental CD spectra[A]//Seijas J A., Pilar Vázquez Tato M. Eleventh International Electronic Conference on Synthetic Organic Chemistry[C]. Switzerland:MDPI, 2007. |
 2016, Vol. 47
2016, Vol. 47


