2. 中国科学院华南植物园, 广东 广州 510650 ;
3. 深圳赛欣瑞科技创新中心, 广东 深圳 518110 ;
4. 北京大学深圳研究院, 广东 深圳 518057
2. South China Botanical Garden, Chinese Academy of Sciences, Guangzhou 510650, China ;
3. Shenzhen Saixinrui Science and Technology Innovation Center, Shenzhen 518110, China ;
4. Shenzhen Graduate School, Peking University Shenzhen 518057, China
桃金娘Rhodomyrtus tomentosa (Ait.) Hassk为桃金娘科(Myrtaceae)桃金娘属Rhodomyrtus (DC.) Reich的常青灌木,别名豆稔、山稔、稔子树等,广泛的分布于东南亚地区,在我国东南部、南部以及西南部均有分布[1]。桃金娘全株都可入药,其叶具有健脾益血和收敛解毒的功效,其根具有祛风行气和益肾的功效[2]。近年来研究表明桃金娘主要化学成分有间苯三酚类、黄酮类、甾体类、蒽类以及三萜类等,这些化合物大多具有较好的生物活性,如抗菌、抗肿瘤、抗氧化等[3-12]。本课题组在寻找具有抗菌活性天然产物过程中发现桃金娘的乙醇提取物具有较强的抗菌效果,为了进一步研究桃金娘的抗菌活性成分,本实验利用活性追踪的方法对桃金娘叶进行了系统的分离,从中分离鉴定了21个化合物,分别为生育酚 [(2R,4'R,8'R)-α-tocopherol,1]、新生育酚 [(2R,4'R,8'R)-β-tocopherol,2]、生育酚-对苯醌(α-tocopherol-quinone,3)、生育酚A(α- tocopherol A,4)、(−)-α-tocospirone(5)、rhodomyrtosone F(6),rhodomyrtosone C(7)、watsonianone A(8)、rhodomyrtone(9)、2,6-二羟基-苯甲酸苯甲酯(verimol K,10)、肉桂酸甲酯(methyl cinnamate,11)、柚皮素(naringenin,12)、槲皮素(quercetin,13)、杨梅素(myricetin,14)、3,7,3′-三甲氧基-5,4′,5′-三羟基黄酮(3,7,3′-trimethoxy-5,4′,5′-trihydroxy flavone,15)、5,7,3′,5′-四羟基黄酮(5,7,3′,5′- tetrahydroxyflavanone,16)、艾纳香素(blumeatin,17)、7,4′-二甲氧基二氢槲皮素(dihydroquercetin- 7,4′-dimethylether,18)、4′-二甲氧基二氢槲皮素(dihydroquercetin-4′-dimethoxy,19)、2,4,7,8,9,10-六羟基-3-甲氧基蒽-6-O-β-L-吡喃鼠李糖苷(2,4,7,8,9,10-hexahydroxy-3-methoxyanthracene-6-O-β-
L-rhamnopyranoside,20)以及4,8,9,10-四羟基-2,3,7-三甲氧基蒽-6-O-β-D-吡喃葡萄糖苷(4,8,9,10- tetrahydroxy-2,3,7-trimethoxyanthracene-6-O-β-D-glucopyranoside,21)。其中化合物6为具有过氧桥键的新天然产物,化合物2~5,10~13以及15~19为首次从该属植物中分离得到。另外,本实验对所有化合物进行了抗菌活性测试,抗菌实验结果显示化合物3及6~9对金黄色葡萄球菌具有一定的抗菌活性。
1 仪器与材料Bruker AV-500 MHz型核磁共振仪(瑞士布鲁克公司);Bruker AV-400 MHz型核磁共振仪(瑞士布鲁克公司);Agilent-1200 Bruker Esquire 6000 Ion Trap液质联用仪(瑞士布鲁克公司);PolAAr 3005旋光仪(OA公司);柱色谱所用填料:硅胶100~200、200~300、300~400目(青岛海洋化工厂分厂),Sephadex LH-20(Pharmacia公司);薄层色谱板所用硅胶为Kieselgel 60 F254、Kieselgel 60、WF254S(Merck公司);色谱用试剂均为分析纯;高效液相试剂均为色谱纯。
桃金娘叶于2013年7月采自江西省南康市,经海南师范大学生命科学技术学院钟琼芯教授鉴定为桃金娘科植物桃金娘Rhodomyrtus tomentosa (Ait.) Hassk。
2 提取分离将采集的桃金娘叶风干,约4.3 kg,粉碎后于95%乙醇中室温浸泡提取3次,每次24 h,合并提取液,并于50 ℃条件下减压浓缩至膏状(400 g)。将所得的乙醇提取物用80~100目硅胶拌样,采用硅胶常压柱依次用石油醚-醋酸乙酯(100∶0→0∶100)和氯仿-甲醇(2∶1→1∶1)进行梯度洗脱。经TLC检测后合并相似流分后得7个组分Fr. 1~7。Fr. 2用硅胶(200~300目)拌样,以石油醚-醋酸乙酯(20∶1→1∶1)进行梯度洗脱,TLC检测后合并相似流分得3个组分Fr. 2.1~2.3;其中Fr. 2.1用凝胶Sephadex LH-20以甲醇-氯仿(1∶1)进行纯化后得到化合物6(18 mg)、11(12 mg)和13(10 mg)。Fr. 2.2经Sephadex LH-20(氯仿-甲醇,1∶1)除去色素后,再由半制备高效液相色谱制备(乙腈-水,48∶52)得到化合物1(6.3 mg)、12(8.1 mg)、15(10 mg)和2(5.8 mg)。Fr. 2.3经Sephadex LH-20凝胶柱色谱以甲醇洗脱后,再由半制备高效液相色谱纯化(乙腈-水,45∶55)得到化合物3(10 mg)及14(5.9 mg)。Fr. 3用硅胶(200~300目)拌样,以石油醚-醋酸乙酯(10∶1→1∶5)进行梯度洗脱,TLC检测后合并相似流分得3个组分Fr. 3.1~3.3,组分Fr. 3.2经Sephadex LH-20凝胶柱色谱以甲醇洗脱后,再由半制备高效液相色谱制备(乙腈-水,40∶60)得到化合物4(9.2 mg)、5(7.8 mg)、16(8.3 mg)和18(8.8 mg)。Fr. 4用硅胶(200~300目)拌样,以石油醚-醋酸乙酯(5∶1→1∶10)进行梯度洗脱,TLC检测后合并相似流分得4个组分Fr. 3.1~3.4;Fr. 3.3经半制备高效液相制备(乙腈-水,35∶65)得到化合物7(6.8 mg)、8(7.1 mg)、9(5.5 mg)、10(9.0 mg)、17(6.6 mg)和19(9.1 mg)。Fr. 5用硅胶(200~300目)拌样,以氯仿-甲醇(50∶1→10∶1)进行梯度洗脱,TLC检测后合并相似流分得2个组分Fr. 5.1~5.2;Fr. 5.1经半制备高效液相制备(乙腈-水,90∶10)得到化合物20(8.8 mg)和21(9.7 mg)。
3 结构鉴定化合物1:淡黄色油状物,EI-MS m/z: 453.4 [M+Na]+。1H-NMR (500 MHz,CDCl3) δ: 2.61 (2H,t,J = 7.0 Hz,H-4),2.16 (3H,s,H-5a),2.12 (6H,s,H-7a,8b),1.23 (3H,s,H-2a),0.87 (6H,d,J = 7.0 Hz,H-4′a,8′a),0.86 (3H,d,J = 5.0 Hz,12′a),0.84 (3H,d,J = 5.0 Hz,12′b);13C-NMR (125 MHz,CDCl3) δ: 74.7 (C-2),31.7 (C-3),20.9 (C-4),118.6 (C-5),144.7 (C-6),121.1 (C-7),122.8 (C-8),117.5 (C-4a),145.7 (C-8a),39.5 (C-1′),21.2 (C-2′),37.6 (C-3′),32.9 (C-4′),37.4 (C-5′),24.6 (C-6′),37.6 (C-7′),32.9 (C-8′),37.6 (C-9′),25.0 (C-10′),40.0 (C-11′),28.1 (C-12′),23.9 (C-2a),11.4 (C-5a),12.4 (C-7a),11.9 (C-8a),19.9 (C-4′a),19.8 (C-8′a),22.9 (C-12′a),22.8 (C-12′a)。以上数据与文献报道一致[13],故鉴定化合物1为生育酚。
化合物2:淡黄色油状物,EI-MS m/z: 439.4 [M+Na]+。1H-NMR (500 MHz,CDCl3) δ: 6.37 (1H,s,H-7),2.67 (2H,dd,J = 11.5,6.5 Hz,H-4),2.14 (3H,s,H-5a),2.12 (3H,s,H-8b),1.25 (3H,s,H-2a),0.86 (6H,d,J = 6.5 Hz,H-4′a,8′a),0.85 (3H,d,J = 5.0 Hz,H-12′a),0.84 (3H,d,J = 5.0 Hz,H-12′b)。该化合物氢谱数据与化合物1极为相似(多出一个芳香质子氢信号,缺少一个单峰甲基氢信号)推测该化合物为新生育酚,对照其主要特征峰与文献报道一致[14],经TLC分析与新生育酚对照品对比鉴定化合物2为新生育酚。
化合物3:淡黄色油状物,EI-MS显示m/z: 469.4 [M+Na]+。1H-NMR (500 MHz,CDCl3) δ: 2.54 (2H,dd,J = 10.5,6.5 Hz,H-1′),2.03 (3H,s,H-3a),2.01 (3H,s,H-5a,6a),1.23 (3H,s,H-3′a),0.87 (6H,d,J = 6.5 Hz,H-7′a,11′a),0.85 (3H,d,J = 6.0 Hz,15′a),0.84 (3H,d,J = 6.0 Hz,15′b);13C-NMR (125 MHz,CDCl3) δ: 187.4 (C-1),144.6 (C-2),140.3 (C-3),187.9 (C-4),140.7 (C-5),140.6 (C-6),12.5 (C-3a),12.5 (C-5a),12.1 (C-6a),21.6 (C-1′),40.4 (C-2′),72.9 (C-3′),42.4 (C-4′),21.5 (C-5′),37.8 (C-6′),33.0 (C-7′),37.6 (C-8′),25.0 (C-9′),37.6 (C-10′),32.9 (C-11′),37.4 (C-12′),24.6 (C-13′),39.5 (C-14′),28.1 (C-15′),22.8 (C-16′),26.7 (C-3′a),19.9 (C-7′a),19.9 (C-11′a),22.9 (C-15′a)。以上数据与文献报道一致[15],故鉴定化合物3为生育酚-对苯醌。
化合物4:无色油状物,EI-MS m/z: 485.4 [M+Na]+。1H-NMR (500 MHz,CDCl3) δ: 4.72 (1H,s,OH),2.43 (1H,dt,J = 12.5,6.0 Hz,Hβ-7),2.01 (3H,s,H-3a),1.90 (1H,dt,J = 12.5,7.0 Hz,Hα-8),1.83 (3H,s,H-6a),1.82 (3H,s,H-5a),1.78 (1H,dt,J = 12.5,7.0 Hz,Hα-7),1.70 (1H,dt,J = 12.5,6.0 Hz,Hβ-8),1.05 (3H,s,H-9a),0.87 (6H,d,J = 6.5 Hz,H-13a,17a),0.86 (3H,d,J = 6.0 Hz,H-21a),0.84 (3H,d,J = 6.0 Hz,H-21b);13C-NMR (125 MHz,CDCl3) δ: 205.1 (C-1),92.3 (C-2),207.3 (C-3),89.2 (C-4),163.2 (C-5),139.5 (C-6),32.9 (C-7),36.3 (C-8),87.2 (C-9),41.6 (C-10),22.6 (C-11),37.6 (C-12),33.0 (C-13),37.7 (C-14),25.0 (C-15),37.7 (C-16),33.0 (C-17),37.4 (C-18),24.6 (C-19),39.5 (C-20),28.1 (C-21),22.9 (C-22),25.0 (C-3a),11.9 (C-5a),8.8 (C-6a),25.6 (C-9a),19.8 (C-13a),19.9 (C-17a),22.8 (C-21a)。以上数据与文献报道一致[16],故鉴定化合物4为生育酚A。
化合物5:无色油状物,EI-MS m/z: 485.4 [M+Na]+。1H-NMR (500 MHz,CDCl3) δ: 3.82 (1H,s,OH),2.06 (3H,s,H-6a),2.05 (3H,s,H-5a),2.03 (1H,m,H-7b),1.94 (1H,m,H-8b),1.69 (1H,m,H-7a),1.66 (1H,m,H-10b),1.60 (1H,m,H-8a),1.58 (1H,m,H-10a),1.36 (3H,s,H-3a),1.33 (3H,s,H-9a),0.87 (6H,d,J = 7.0 Hz,H-13a,17a),0.85 (3H,d,J = 5.5 Hz,H-21a),0.84 (3H,d,J = 5.5 Hz,21b);13C-NMR (125 MHz,CDCl3) δ: 199.0 (C-1),93.5 (C-2),81.4 (C-3),201.8 (C-4),142.1 (C-5),147.1 (C-6),32.2 (C-7),36.6 (C-8),87.2 (C-9),41.5 (C-10),22.5 (C-11),37.6 (C-12),32.9 (C-13),37.6 (C-14),24.9 (C-15),37.4 (C-16),32.9 (C-17),37.4 (C-18),24.6 (C-19),39.5 (C-20),28.1 (C-21),22.8 (C-22),24.4 (C-3a),13.2 (C-5a),13.6 (C-6a),25.9 (C-9a),19.9 (C-13a),19.9 (C-17a),22.8 (C-21a)。以上数据与文献报道一致[17],故鉴定化合物5为(−)-α-tocospirone。
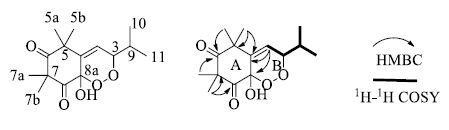
化合物6:白色粉末状,EI-MS m/z: 305.1 [M+Na]+,HR-ESI-MS显示m/z: 305.136 8 [M+Na]+结合碳谱可以确定该化合物的分子式为C15H22O5(不饱和度为5)。1H-NMR (CDCl3,500 MHz) 谱中(表 1)显示存在1个烯氢信号δ: 7.29 (1H,d,J = 1.5 Hz,H-4),1个连氧次甲基氢信号4.73 (1H,dd,J = 6.0,1.5 Hz,H-3) 以及1个次甲基氢信号2.01 (1H,m,H-9),此外还有4个甲基的单峰氢信号分别为δ 1.39 (3H,s),1.37 (3H,s),1.31 (3H,s),1.02 (3H,s),以及2个化学位移相同的甲基双峰氢信号1.05 (6H,d,J = 7.0 Hz)。13C-NMR (CDCl3,125 MHz) 结合DEPT谱可知该化合物中存在2个羰基碳信号δ 198.0 (C-6) 及210.7 (C-8);1对双键碳信号δ 138.1 (C-4) 及134.5 (C-4a);1个连二氧饱和碳信号δ 98.1 (C-8a);1个连氧碳信号δ 83.7 (C-3);2个饱和季碳信号δ 51.9 (C-5) 及55.1 (C-7);1个饱和次甲基碳信号δ 30.7 (C-9) 以及余下6个甲基碳信号分别为δ 26.8,24.2,20.8,18.4,16.6以及15.3。以上数据说明该化合物为间苯三酚类衍生物。HMBC谱中显示H-5a,5b与C-4a,5,6相关;H-7a,7b与C-6,7,8位碳相关;H-4与C-4a,5,8a相关可以说明A环的连接方式(图 1)。1H-1H COSY谱中显示H-11,10与H-9,H-3,H-4有相关说明存在如图 1中加粗部分的片段,该片段连在C-4a位是通过HMBC相关进一步确定的。从3位和8位碳的化学位移和高分辨质谱给出的分子式可以推断出B环的连接方式。因此确定该化合物的结构如图 1所示,并将化合物6命名为rhodomyrtosone F。该化合物仅作为合成的中间产物被报道过[18],而从未从植物中分离得到,因此化合物6为1个新的天然产物。
|
|
表 1 化合物6 1H-NMR和3C-NMR数据 Table 1 1H-NMR and 13C-NMR data for compound 6 |
|
图 1 化合物6的结构及重要的HMBC、1H-1H COSY相关 Fig.1 Structure and key correlations of HMBC and 1H-1H COSY for compound 6 |
化合物7:黄色粉末,EI-MS m/z: 697.4 [M+Na]+。1H-NMR (500 MHz,CDCl3) δ: 13.5 (1H,s,8-OH),4.39 (1H,t,J = 3.5 Hz,H-7″),4.35 (1H,t,J = 5.5,H-9),3.21 (1H,dd,J = 17.5,7.0 Hz,H-2′a),3.00 (1H,dd,J = 17.5,6.5 Hz,H-2′b),1.65 (3H,s,H-14′′′),1.62 (3H,s,H-12),1.51 (3H,s,H-15′′′),1.47 (3H,s,H-13),1.43 (3H,s,H-12′′′),1.41 (3H,s,H-13′′′),1.36 (3H,s,H-3a),1.39 (3H,s,H-10),1.36 (3H,s,H-11),1.03 (3H,d,J = 6.0 Hz,H-4′),1.02 (3H,d,J = 6.5 Hz,H-5′),0.96 (3H,d,J = 6.0 Hz,H-11′′′),0.89 (3H,d,J = 6.0 Hz,H-4″),0.82 (3H,d,J = 6.5 Hz,H-10′′′);13C-NMR (125 MHz,CDCl3) δ: 197.6 (C-1),56.2 (C-2),211.8 (C-3),47.4 (C-4),166.8 (C-4a),152.5 (C-4b),105.8 (C-5),150.6 (C-6),107.7 (C-7),160.8 (C-8),107.7 (C-8a),25.7 (C-9),114.4 (C-9a),25.0 (C-10),24.5 (C-11),24.8 (C-12),25.1 (C-13),204.7 (C-1′),54.1 (C-2′),24.7 (C-3′),25.2 (C-4′),22.8 (C-5′),45.6 (C-1″),25.4 (C-2″),23.4 (C-3″),23.5 (C-4″),197.6 (C-1′′′),56.4 (C-2′′′),211.6 (C-3′′′),47.4 (C-4′′′),166.9 (C-5′′′),113.8 (C-6′′′),25.4 (C-7′′′),47.0 (C-8′′′),25.1 (C-9′′′),23.5 (C-10′′′),23.9 (C-11′′′),24.3 (C-12′′′),24.0 (C-13′′′),25.1 (C-14′′′)。以上数据与文献报道一致[19],故鉴定化合物7为rhodomyrtosone C。
化合物8:黄色粉末,EI-MS m/z: 455.2 [M+Na]+。1H-NMR (500 MHz,CDCl3) δ: 13.3 (2H,s,5,5″-OH),4.09 (1H,t,J = 7.5,H-1′),1.82 (2H,t,J = 7.5,H-2′),1.43 (6H,s,H-9,9″),1.43 (6H,s,H-8,8″),1.39 (1H,m,H-3′) 1.37 (6H,s,H-7,7″),1.36 (6H,s,H-10,10″),0.84 (6H,d,J = 6.5 Hz,H-4′,5′);13C-NMR (125 MHz,CDCl3) δ: 191.8 (C-1,1″),52.3 (C-2,2″),212.5 (C-3,3″),51.6 (C-4,4″),191.5 (C-5,5″),114.2 (C-6,6″),24.2 (C-7,7″),26.1 (C-8,8″),25.1 (C-9,9″),25.4 (C-10,10″),29.7 (C-1′),38.0 (C-2′),27.0 (C-3′),22.5 (C-4′,5′)。以上数据与文献报道一致[20],故鉴定化合物8为watsonianone A。
化合物9:黄色粉末,EI-MS m/z: 465.2 [M+Na]+。1H-NMR (500 MHz,CD3OD) δ: 6.13 (1H,s,H-5),4.20 (1H,t,J = 6.5,H-9),3.03 (1H,dd,J = 15.5,6.5 Hz,H-2′a),2.97 (1H,dd,J = 15.5,7.0 Hz,H-2′b),2.25 (1H,m,H-3′),1.56 (3H,s,H-12),1.44 (3H,s,H-13),1.42 (3H,s,H-10),1.38 (3H,s,H-11),0.98 (3H,d,J = 6.5 Hz,H-4′),0.98 (3H,d,J = 7.0 Hz,H-5′),0.87 (3H,d,J = 6.0 Hz,H-4″),0.84 (3H,d,J = 5.5 Hz,H-5″);13C-NMR (125 MHz,CD3OD) δ: 199.5 (C-1),57.1 (C-2),213.5 (C-3),48.5 (C-4),168.9 (C-4a),157.4 (C-4b),95.2 (C-5),164.2 (C-6),108.9 (C-7),161.8 (C-8),106.1 (C-8a),26.5 (C-9),115.3 (C-9a),26.2 (C-10),24.1 (C-11),24.9 (C-12),24.3 (C-13),208.6 (C-1′),57.1 (C-2′),26.4 (C-3′),25.4 (C-4′),23.7 (C-5′),46.5 (C-1″),26.4 (C-2″),23.1 (C-3″),24.3 (C-4″)。以上数据与文献报道一致[3],故鉴定化合物9为rhodomyrtosone。
化合物10:无色油状物,EI-MS m/z: 267.1 [M+Na]+。1H-NMR (500 MHz,CDCl3) δ: 9.70 (2H,brs,1′,3′-OH),7.43 (5H,m,H-3~7),7.31 (1H,t,J = 8.0 Hz,H-5′),6.47 (2H,d,J = 8.0 Hz,H-4′,6′),2.25 (1H,m,H-3′),5.50 (2H,s,H-1);13C-NMR (125 MHz,CDCl3) δ: 68.4 (C-1),134.0 (C-2),128.9 (C-3,7),129.2 (C-4,6),129.5 (C-5),161.1 (C-1′,3′),100.2 (C-2′),108.4 (C-4′,6′),136.9 (C-5′),169.6 (C-7′)。以上数据与文献报道一致[21],故鉴定化合物10为2,6-二羟基-苯甲酸苯甲酯。
化合物11:无色针状结晶(甲醇),EI-MS m/z: 163.1 [M+H]+。1H-NMR (500 MHz,CDCl3) δ: 7.69 (H,d,J = 16.0 Hz,H-2),7.52 (2H,m,H-5,9),7.38 (3H,m,H-6~8),6.44 (H,d,J = 16.0 Hz,H-3),3.81 (3H,s,OMe);13C-NMR (125 MHz,CDCl3) δ: 167.5 (C-1),117.9 (C-2),144.9 (C-3),134.5 (C-4),128.2 (C-5,9),128.9 (C-6,8),130.4 (C-7),51.8 (OMe)。以上数据与文献报道一致[22],故鉴定化合物11为肉桂酸甲酯。
化合物12:白色针晶(甲醇),mp 260~262 ℃,EI-MS m/z: 295.1 [M+Na]+。1H-NMR (400 MHz,CD3OD-d4) δ: 2.68 (1H,dd,J = 13.6,2.4 Hz,H-3a),3.10 (1H,dd,J = 13.6,10.4 Hz,H-3b),5.32 (1H,dd,J = 10.4,2.4 Hz,H-2),5.88 (1H,d,J = 2.0 Hz,H-6),5.89 (1H,d,J = 2.0 Hz,H-8),6.81 (2H,d,J = 8.4 Hz,H-3′,5′),7.30 (2H,d,J = 8.4 Hz,H-2′,6′);13C-NMR (100 MHz,CD3OD-d4) δ: 44.0 (C-3),80.5 (C-2),96.2 (C-8),97.0 (C-6),103.3 (C-10),116.3 (C-3′,5′),129.0 (C-2′,6′),131.1 (C-1′),159.0 (C-4′),164.9 (C-9),165.5 (C-5),168.3 (C-7),197.8 (C-4)。以上数据与文献报道一致[23],故鉴定化合物12为柚皮素。
化合物13:黄色针晶(甲醇),mp 308~309 ℃,EI-MS m/z: 325.0 [M+Na]+。1H-NMR (400 MHz,CD3COCD3) δ: 6.27 (1H,d,J = 1.6 Hz,H-6),6.54 (1H,d,J = 1.6 Hz,H-8),7.00 (1H,d,J = 8.4 Hz,H-5′),7.70 (1H,dd,J = 8.4,1.6 Hz,H-6′),7.83 (1H,d,J = 1.6 Hz,H-2′);13C-NMR (100 MHz,CD3COCD3) δ: 94.4 (C-8),99.0 (C-6),104.0 (C-10),115.7 (C-5′),116.2 (C-2′),121.4 (C-6′),123.7 (C-1′),136.7 (C-3),145.7 (C-3′),146.9 (C-2),148.2 (C-4′),157.8 (C-9),162.0 (C-5),164.9 (C-7),176.5 (C-4)。以上数据与文献报道一致[24],故鉴定化合物13为槲皮素。
化合物14:黄色针晶(甲醇),mp 323~324 ℃,EI-MS m/z: 341.0 [M+Na]+。1H-NMR (400 MHz,CD3OD-d4) δ: 6.18 (1H,d,J = 1.6 Hz,H-6),6.38 (1H,d,J = 1.6 Hz,H-8),7.35 (2H,s,H-2′,6′);13C-NMR (100 MHz,CD3OD-d4) δ: 94.4 (C-8),99.2 (C-6),104.5 (C-10),108.5 (C-2′,6′),123.1 (C-1′),137.0 (C-3),137.4 (C-4′),146.7 (C-3′,5′),148.0 (C-2),158.2 (C-9),162.5 (C-5),165.6 (C-7),177.3 (C-4)。以上数据与文献报道一致[25],故鉴定化合物14为杨梅素。
化合物15:黄色针晶(甲醇),mp 198~199 ℃,EI-MS m/z: 383.1 [M+Na]+。1H-NMR (400 MHz,CD3OD-d4) δ: 3.80 (3H,s,3-OMe),3.87 (3H,s,7-OMe),3.91 (3H,s,3′-OMe),6.29 (1H,d,J = 1.6 Hz,H-6),6.55 (1H,d,J = 1.6 Hz,H-8),7.31 (1H,d,J = 1.6 Hz,H-6′),7.34 (1H,d,J = 1.6 Hz,H-3′);13C-NMR (100 MHz,CD3OD-d4) δ: 93.1 (C-8),98.9 (C-6),105.3 (C-10),106.7 (C-2′),110.9 (C-6′),121.7 (C-1′),139.1 (C-4′),139.9 (C-3),146.6 (C-5′),149.5 (C-3′),158.1 (C-2),158.2 (C-9),162.8 (C-5),167.3 (C-7),180.0 (C-4)。以上数据与文献报道一致[26],故鉴定化合物15为3,7,3′-三甲氧基-5,4′,5′-三羟基黄酮。
化合物16:白色粉末(甲醇),mp 265~266 ℃,EI-MS m/z: 311.1 [M+Na]+。1H-NMR (400 MHz,DMSO-d6) δ: 2.66 (1H,dd,J = 14.4,2.4 Hz,H-3a),3.17 (1H,dd,J = 14.4,12.0 Hz,H-3b),5.35 (1H,dd,J = 12.0,2.4 Hz,H-2),5.88 (2H,s,H-6,8),6.75 (2H,s,H-2′,6′),6.88 (1H,s,H-4′);13C-NMR (100 MHz,DMSO-d6) δ: 42.0 (C-3),78.6 (C-2),95.1 (C-8),95.8 (C-6),101.9 (C-10),114.5 (C-2′),115.2 (C-6′),118.1 (C-4′),129.6 (C-1′),145.2 (C-3′),145.9 (C-5′),163.1 (C-5),163.8 (C-9),166.7 (C-7),196.4 (C-4)。以上数据与文献报道一致[27],故鉴定化合物16为5,7,3′,5′-四羟基黄酮。
化合物17:白色粉末(甲醇),mp 217~218 ℃,EI-MS m/z: 325.1 [M+Na]+。1H-NMR (400 MHz,DMSO-d6) δ: 2.71 (1H,dd,J = 14.0,2.4 Hz,H-3a),3.23 (1H,dd,J = 14.0,11.2 Hz,H-3b),3.79 (3H,s,7-OMe),5.44 (1H,dd,J = 11.2,2.4 Hz,H-2),6.08 (1H,d,J = 2.4 Hz,H-6),6.11 (1H,d,J = 2.4 Hz,H-8),6.75 (2H,s,H-2′,6′),6.89 (1H,s,H-4′);13C-NMR (100 MHz,DMSO-d6) δ: 42.1 (C-3),78.9 (C-2),93.5 (C-8),94.4 (C-6),102.6 (C-10),114.2 (C-2′),115.5 (C-6′),118.3 (C-4′),129.5 (C-1′),145.3 (C-3′),145.9 (C-5′),162.8 (C-5),163.4 (C-9),167.8 (C-7),192.8 (C-4),56.1 (7-OMe)。以上数据与文献报道一致[27],故鉴定化合物17为艾纳香素。
化合物18:白色粉末(甲醇),mp 165~167 ℃,EI-MS m/z: 355.2 [M+Na]+。1H-NMR (400 MHz,DMSO-d6) δ: 3.78 (3H,s,4′-OMe),3.81 (3H,s,7-OMe),4.58 (1H,d,J = 11.2 Hz,H-3),5.18 (1H,d,J = 11.2 Hz,H-2),6.08 (1H,d,J = 2.4 Hz,H-6),6.10 (1H,d,J = 2.4 Hz,H-8),6.91 (1H,dd,J = 8.0,2.4 Hz,H-6′),6.93 (1H,d,J = 2.4 Hz,H-2′),7.06 (1H,d,J = 8.0 Hz,H-5′);13C-NMR (100 MHz,DMSO-d6) δ: 71.6 (C-3),83.1 (C-2),93.7 (C-8),94.9 (C-6),101.8 (C-10),111.8 (C-5′),115.3 (C-2′),119.3 (C-6′),129.5 (C-1′),145.9 (C-3′),148.2 (C-4′),162.6 (C-9),163.2 (C-5),167.8 (C-7),198.2 (C-4),56.1 (7-OMe),55.9 (4′-OMe)。以上数据与文献报道一致[27],故鉴定化合物18为7,4′-二甲氧基二氢槲皮素。
化合物19:白色粉末(甲醇),mp 175~176 ℃,EI-MS m/z: 341.2 [M+Na]+。1H-NMR (400 MHz,CD3OD-d4) δ: 3.89 (3H,s,4′-OMe),4.58 (1H,d,J = 9.2 Hz,H-3),4.98 (1H,d,J = 9.2 Hz,H-2),5.90 (1H,d,J = 1.6 Hz,H-6),5.93 (1H,d,J = 1.6 Hz,H-8),6.83 (1H,d,J = 6.4 Hz,H-5′),6.97 (1H,dd,J = 6.4,1.6 Hz,H-6′),7.11 (1H,d,J = 1.6 Hz,H-2′);13C-NMR (100 MHz,CD3OD-d4) δ: 74.0 (C-3),85.3 (C-2),97.2 (C-8),98.5 (C-6),102.8 (C-10),114.8 (C-5′),117.3 (C-2′),121.3 (C-6′),132.1 (C-1′),148.9 (C-3′),150.0 (C-4′),164.6 (C-9),165.3 (C-5),169.8 (C-7),199.2 (C-4),58.2 (4′-OMe)。以上数据与文献报道一致[27],故鉴定化合物19为4′-二甲氧基二氢槲皮素。
化合物20:黄色粉末(甲醇),EI-MS m/z: 489.4 [M+Na]+。1H-NMR (400 MHz,Pyridine-d5) δ: 1.68 (3H,d,J = 5.2 Hz,H-6′),4.44 (1H,m,H-4′),4.62 (1H,m,H-3′),4.79 (1H,m,H-5′),4.94 (1H,m,H-2′),4.21 (3H,s,3-OMe),6.45 (1H,d,J = 1.2 Hz,H-1′),8.06 (1H,s,H-1),8.50 (1H,s,H-5);13C-NMR (100 MHz,Pyridine-d5) δ: 18.9 (C-6′),71.8 (C-5′),72.2 (C-3′),72.9 (C-2′),74.1 (C-4′),102.5 (C-1′),108.1 (C-8a),112.5 (C-9a),113.1 (C-1),114.6 (C-5),115.2 (C-4a),115.8 (C-10a),137.9 (C-7),141.6 (C-3),143.1 (C-4,8),148.3 (C-6),154.6 (C-2),159.8 (C-10),160.1 (C-9),61.6 (3-OMe)。以上数据与文献报道一致[8],故鉴定化合物20为2,4,7,8,9,10-六羟基-3-甲氧基蒽-6-O-β-L-吡喃鼠李糖苷。
化合物21:黄色粉末(甲醇),EI-MS m/z: 533.3 [M+Na]+。1H-NMR (400 MHz,DMSO-d6) δ: 3.23 (1H,m,H-4′),3.34 (2H,m,H-2′,3′),3.42 (1H,m,H-5′),3.51 (1H,d,J = 11.2 Hz,H-6′a),3.70 (1H,d,J = 11.2 Hz,H-6′b),4.00 (3H,s,2-OMe),4.04 (3H,s,3-OMe),4.10 (3H,s,7-OMe),5.18 (1H,d,J = 7.6 Hz,H-1′),7.63 (1H,s,H-1),7.83 (1H,s,H-5);13C-NMR (100 MHz,DMSO-d6) δ: 60.6 (C-6′),69.5 (C-4′),73.4 (C-2′),76.5 (C-3′),77.3 (C-5′),101.3 (C-1′),107.6 (C-1),112.1 (C-5),112.4 (C-8a),112.7 (C-4a),112.9 (C-9a),113.7 (C-10a),141.0 (C-3),141.2 (C-4,8),141.8 (C-7),151.9 (C-6),154.4 (C-2),158.2 (C-10),158.4 (C-9),56.8 (2-OMe),61.4 (3-OMe),61.7 (7-OMe)。以上数据与文献报道一致[8],故鉴定化合物21为4,8,9,10-四羟基-2,3,7-三甲氧基蒽-6-O-β-D-吡喃葡萄糖苷。
4 抗菌活性采用微量稀释法[28]对分离得到的化合物1~21进行抗菌活性测试。供试菌株为大肠杆菌Escherichia coli ATCC 25922和金黄色葡萄球菌Staphyloccocus aureus ATCC 8799,阳性对照为红霉素,其对大肠杆菌和金黄色葡萄球菌的MIC值分别为125 μg/mL和0.63 μg/mL。结果表明化合物9对金黄色葡萄球菌有较强的抑制活性,其MIC为1.25 μg/mL,接近阳性对照组,化合物3以及6~8则对金黄色葡萄球菌具有一定的抑制活性,其MIC值分别为10、10、2.5和5 μg/mL ;其他化合物在20 μg/mL时对2种致病菌都未见有明显抑制活性。
| [1] | Winotai A, Wright T, Goolsby A J. Herbivores in Thailand on Rhodomyrtus tomentosa (Myrtaceae), an invasive weed in Florida[J]. Fla Entomol , 2005, 88 (1) :104–105. |
| [2] | 国家中医药管理局中华本草编委会. 中华本草[M]. 上海: 上海科学技术技术社, 1999 . |
| [3] | Hiranrat A, Mahabusarakam W. New acylphloroglucinols from the leaves of Rhodomyrtus tomentosa[J]. Tetrahedron , 2008, 64 (49) :11193–11197. |
| [4] | Hiranrat A, Chitbankluoi W, Mahabusarakam W, et al. A new flavellagic acid derivative and phloroglucinol from Rhodomyrtus tomentosa[J]. Nat Prod Res , 2012, 26 (20) :1904–1909. |
| [5] | Hiranrat A, Mahabusarakam W, Carroll A R, et al. Tomentosones A and B, hexacyclic phloroglucinol derivatives from the Thai shrub Rhodomyrtus tomentosa[J]. J Org Chem , 2012, 77 (1) :680–683. |
| [6] | 侯爱君, 吴养洁, 刘延泽. 桃金娘中的黄酮苷和一种逆没食子丹宁[J]. 中草药 , 1999, 30 (9) :645–648. |
| [7] | Hui W H, Li M M, Luk K. Triterpenoids and steroids from Rhodomyrtus tomentosa[J]. Phytochemistry , 1975, 14 (3) :833–834. |
| [8] | Tung N H, Ding Y, Choi E M, et al. New anthracene glycosides from Rhodomyrtus tomentosa stimulate osteoblastic differentiation of MC3T3-E1 cells[J]. Arc Pharm Res , 2009, 32 (4) :515–520. |
| [9] | 黄敏琪, 牙启康, 陈海燕, 等. 山菍茎枝化学成分的研究[J]. 中草药 , 2010, 41 (7) :1079–1080. |
| [10] | Takahashi H, Iuchi M, Fujita Y, et al. Coumaroyl triterpenes from Casuarina equisetifolia[J]. Phytochemistry , 1999, 51 (4) :543–550. |
| [11] | Hui W H, Li M M, Luk K. Triterpenoids and steroids from Rhodomyrtus tomentosa[J]. Phytochemistry , 1975, 14 (3) :833–834. |
| [12] | 肖婷, 崔炯谟, 李倩, 等. 桃金娘的化学成分、药理作用和临床应用研究进展[J]. 现代药物与临床 , 2013, 28 (5) :800–805. |
| [13] | Kitajima J, Kimizuka K, Arai M, et al. Constiuents of Ficus pumila leaves[J]. Chem Pharm Bull , 1998, 46 (10) :1647–1649. |
| [14] | 夏芳, 孙建, 姜勇, 等. 白木香叶的化学成分研究(二)[J]. 中国中药杂志 , 2013, 38 (19) :3299–3303. |
| [15] | Sung J H, Lee J O, Son J K, et al. Cytotoxic constituents from Solidago virga-aurea var. gigantean MIQ[J]. Arch Pharm Res , 1999, 22 (6) :633–637. |
| [16] | Chiang Y M, Kuo Y H. Two novel α-tocopheroids from the aerial roots of Ficus microcarpa[J]. Tetrahedron Lett , 2003, 44 (27) :5125–5128. |
| [17] | Lin W Y, Kuo Y H, Chang Y L, et al. Anti-platelet aggregation and chemical constituents from the rhizome of Gynura japonica[J]. Planta Med , 2003, 69 (8) :757–764. |
| [18] | Gervais A, Lazarski K E, Porco J A. Divergent total syntheses of rhodomyrtosones A and B[J]. J Org Chem , 2015, 80 (19) :9584–9591. |
| [19] | Carroll A R, Avery V M, Duffy S, et al. Watsonianone A-C, anti-plasmodial β-triketones from the Australian tree, Corymbia watsoniana[J]. Org Biomol Chem , 2013, 11 (3) :453–458. |
| [20] | Hamidi D, Salni S, Sargent M V, et al. Rhodomyrtone, an antibiotic from Rhodomytus tomentosa[J]. Chem Inform , 2002, 33 (45) :229–232. |
| [21] | Sy L K, Brown G D. Novel phenylpropanoids and lignans from Illicium verum[J]. J Nat Prod , 1998, 61 (8) :987–992. |
| [22] | Reta G F, Tonn C E, Ríos-Luci C, et al. Cytotoxic bioactivity of some phenylpropanoic acid derivatives[J]. Nat Prod Commun , 2012, 7 (10) :1341–1346. |
| [23] | 官智, 谭颂德, 苏镜娱, 等. 云南多依(Docynia delavayi (Franch.) Schneid.)黄酮成分研究[J]. 天然产物研究与开发 , 2000, 12 (3) :34–37. |
| [24] | Liu R H, Wen X C, Shao F, et al. Flavonoids from heartwood of Dalbergia cochinchinensis[J]. Chin Herb Med , 2016, 8 (1) :89–93. |
| [25] | 周先礼, 亲长红, 梅莹, 等. 髯花杜鹃叶的化学成分研究[J]. 中草药 , 2010, 41 (2) :206–208. |
| [26] | Kumari G N K, Rao L J M, Rao N S P. Myricetin methyl ethers from Solanum pubescens[J]. Phytochemistry , 1984, 23 (11) :2701–2702. |
| [27] | Nessa F, Ismail Z, Mohamed N, et al. Free radicalscavenging activity of organic extracts and of pure flavonoids of Blumea balsamifera DC[J]. Food Chem , 2004, 88 (2) :243–252. |
| [28] | Pierce C G, Uppuluri P, Tristan A R, et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing[J]. Nat Protoc , 2008, 3 (9) :1494–1500. |
 2016, Vol. 47
2016, Vol. 47


