鄂西大蓟Cirsium henryi (Franch.) Diels系菊科(Compositae)蓟属Cirsium Mill. Emend. Scop. 多年生草本植物,又名刺包蓟,以根和茎叶入药。该植物广泛分布于鄂西山区,具有凉血止血、祛瘀消肿之功效,民间用于治疗咯血、便血、尿血、崩漏下血、瘀肿疮毒等症[1-2]。已有研究报道该属植物中含有三萜、甾醇、黄酮、黄酮苷、长链炔烯醇和挥发油[3]等化学成分。本课题组前期从鄂西大蓟根中分离鉴定了10个化合物,其中乙酰丁香酸(p-hydroxycinnamic acid)和香草酸(vanilIic acid)对小鼠肿瘤细胞S180增殖具有显著的抑制作用[4-5]。为了更加深入地研究鄂西大蓟的化学成分,阐明其药效学物质基础,本实验对鄂西大蓟茎叶的化学成分进行了系统研究,从中分离鉴定了16个化合物,分别为硬脂酸(stearic acid,1)、二羟丙基软脂酸酯(2,3-dihydroxypropyl hexadecanoate,2)、软脂酸(palmitic acid,3)、蒲公英甾醇(taraxasterol,4)、伪蒲公英甾醇(pseudo taraxasterol,5)、蒲公英甾醇乙酸酯(taraxasterol acetate,6)、β-谷甾醇(β-sitosterol,7)、胡萝卜苷(daucosterol,8)、原儿茶酸(protocatechuic acid,9)、尿嘧啶(uracil,10)、蒙花苷(linarin,11)、芹菜素(apigenin,12)、槲皮苷(quercitrin,13)、金合欢素(acacetin,14)、金合欢素-7-O-β-D-葡萄糖苷(acacetin-7-O-β-D-glucoside,15)、4-羟基-3,5-二甲氧基苯甲酸(4-hydroxy-3,5-dimethoxy benzoic acid,16)。化合物1~16的结构见图 1。其中化合物3、10~16为首次从该植物中分离得到,化合物10、13、16为首次从蓟属植物中分离得到。
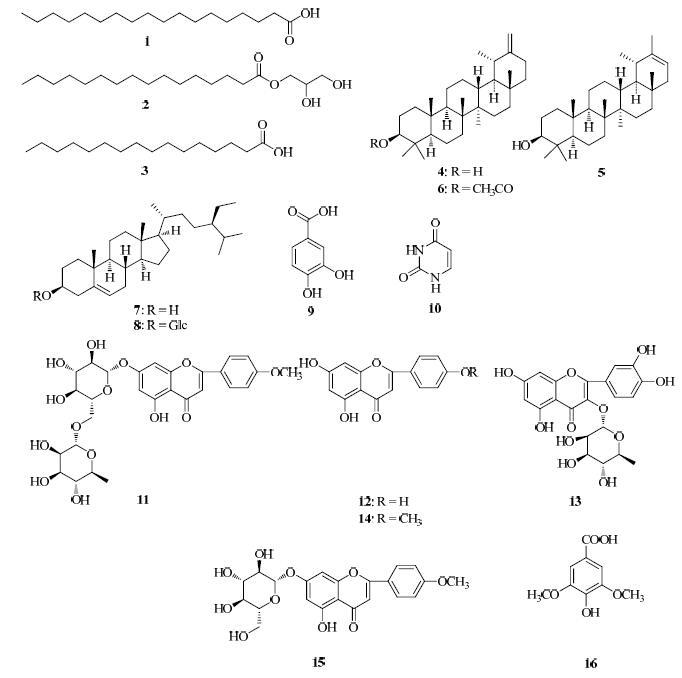
|
图 1 化合物1~16的结构 Fig.1 Structures of compounds 1—16 |
1 仪器与材料
Bruker AM-400 MHz核磁共振仪(Bruker公司);HR-ESI-MS质谱仪(赛默飞公司);X4型显微熔点仪(北京泰克仪器有限公司);旋转蒸发仪(上海亚荣生化仪器厂);柱色谱硅胶(200~300目)和薄层色谱硅胶GF254(青岛海洋化工);所用试剂均为分析纯。
鄂西大蓟于2004年11月采集于湖北神农架地区,经神农架药检所石世贵主任药师鉴定为菊科管状花亚科菜蓟族蓟属植物鄂西大蓟Cirsium henryi (Franch.) Diels,植物标本现保存于华中科技大学同济医学院天然药物化学教研室,标本号为20041101。
2 提取与分离鄂西大蓟茎叶20 kg,阴干后粉碎,用95%乙醇渗漉提取4次,合并提取液,滤液减压浓缩,得到乙醇浸膏376 g,将浸膏混悬于温水后,依次用石油醚、醋酸乙酯、正丁醇萃取,回收溶剂后得到石油醚部分120 g,醋酸乙酯部分86 g,正丁醇部分40 g。石油醚部分经硅胶柱色谱分离,石油醚-醋酸乙酯(100∶0→0∶50)和醋酸乙酯-甲醇(50∶0→0∶100)为洗脱剂梯度洗脱得到4个流分Fr. 1.1~Fr. 1.4。Fr. 1.1(3.5 g)经硅胶柱色谱分离,石油醚-醋酸乙酯(100∶0→10∶90)得到化合物1(17.5 mg)、2(19 mg);Fr. 1.2(6.8 g)经硅胶柱色谱分离,石油醚-醋酸乙酯(100∶0→10∶90)洗脱得到化合物3(1.2 g);Fr. 1.3(5.4 g)经硅胶柱色谱分离,石油醚-醋酸乙酯(100∶0→5∶95)洗脱得到化合物4(200 mg)、5(32 mg)、6(25 mg);Fr. 1.4(7.6 g)经重结晶得到化合物7(367 mg)。醋酸乙酯部分经硅胶色谱分离,石油醚-醋酸乙酯(100∶0→10∶90)梯度洗脱,合并相同流分,再经硅胶柱色谱反复分离纯化得到化合物8(120 mg)、9(13 mg)、11(22 mg)、13(65 mg)、14(32 mg)。正丁醇部分经硅胶色谱分离,石油醚-醋酸乙酯(100∶0→10∶90)以及醋酸乙酯-甲醇(100∶0→10∶90)梯度洗脱,合并相同流分,再经硅胶柱色谱反复分离及纯化得到化合物10(21 mg)、12(17 mg)、15(27 mg)、16(22 mg)。
3 结构鉴定化合物1:白色粉末,mp 67~69 ℃。1H-NMR (400 MHz,CDCl3) δ: 2.31 (2H,t,J = 7.8 Hz,H-2),1.63 (2H,m,H-3),1.28 (28H,m,H-4~17),0.88 (3H,t,J = 7.2 Hz,H-18);13C-NMR (100 MHz,CDCl3) δ: 180.1 (C-1),34.0 (C-2),31.9 (C-16),29.7,29.6,29.4,29.4,29.2,29.1 (多个亚甲基C信号,C-4~15),24.7 (C-3),22.7 (C-17),14.51 (C-18)。以上数据与文献报道基本一致[6],故鉴定化合物1为硬脂酸。
化合物2:白色颗粒。1H-NMR (400 MHz,CDCl3) δ: 4.20 (1H,m,H-1a),4.10 (1H,m,H-1b),3.96 (1H,m,H-2),3.72 (1H,m,H-3a),3.65 (1H,dd,J = 11.0,4.0 Hz,H-3b),2.40 (2H,t,J = 7.0 Hz,H-2′),1.64 (2H,m,H-3′),1.21~1.27 (24H,m,H-4′~H-15′),0.90 (3H,t,J = 7.0 Hz,H-16′);13C-NMR (100 MHz,CDCl3) δ: 174.4 (C-1′),70.3 (C-2),65.2 (C-1),63.4 (C-3),34.2 (C-2′),32.0 (C-3′),29.7,29.6,29.5,29.4,29.3,29.2 (多个亚甲基C信号,C-4′~13′),25.0 (C-14′),22.7 (C-15′),14.1 (C-16′)。以上数据与文献报道基本一致[7],故鉴定化合物2为二羟丙基软脂酸酯。
化合物3:白色粉末。1H-NMR (400 MHz,CDCl3) δ: 2.25 (2H,t,J = 7.8 Hz,H-2),1.61 (2H,m,H-3),1.31 (24H,m,H-4~15),0.92 (3H,t,J = 7.2 Hz,H-16);13C-NMR (100 MHz,CDCl3) δ: 179.5 (C-1),36.4 (C-2),24.9 (C-3),29.9,29.8,29.6,29.5 (多个亚甲基C信号,C-4~13),31.9 (C-14),23.6 (C-15),14.5 (C-16)。以上数据与文献报道基本一致[8],故鉴定化合物3为软脂酸。
化合物4:无色粉末。1H-NMR (400 MHz,CDCl3) δ: 4.61 (2H,m,H-30),3.20 (1H,dd,J = 10.2,2.4 Hz,H-3),0.96 (3H,d,J = 7.0 Hz,H-29),1.04 (3H,s,H-26),0.90 (3H,s,H-27),1.02 (3H,s,H-23),0.93 (3H,s,H-28),0.83 (3H,s,H-25),0.77 (3H,s,H-24);13C-NMR (100 MHz,CDCl3) δ: 38.8 (C-1),26.9 (C-2),79.2 (C-3),38.9 (C-4),55.3 (C-5),18.0 (C-6),33.8 (C-7),40.0 (C-8),50.1 (C-9),37.2 (C-10),21.4 (C-11),26.8 (C-12),38.8 (C-13),42.3 (C-14),26.9 (C-15),38.3 (C-16),33.2 (C-17),48.7 (C-18),39.4 (C-19),154.7 (C-20),35.4 (C-21),38.8 (C-22),28.0 (C-23),15.9 (C-24),16.8(C-25),16.3 (C-26),14.8 (C-27),19.5 (C-28),25.6 (C-29),107.1 (C-30)。以上数据与文献报道基本一致[9],故鉴定化合物4为蒲公英甾醇。
化合物5:无色粉末,mp 196~198 ℃。1H-NMR (400 MHz,CDCl3) δ: 4.61 (2H,m,H-30),3.20 (1H,dd,J = 10.2,2.4 Hz,H-3),1.63 (3H,s,H-29),1.04 (3H,s,H-26),1.02 (3H,s,H-23),0.93 (3H,s,H-28),0.90 (3H,s,H-27),0.83 (3H,s,H-25),0.76 (3H,s,H-24);13C-NMR (100 MHz,CDCl3) δ: 38.8 (C-1),27.4 (C-2),79.0 (C-3),40.1 (C-4),55.2 (C-5),21.4 (C-6),34.4 (C-7),41.1 (C-8),50.4 (C-9),38.8 (C-10),21.6 (C-11),28.0 (C-12),39.4 (C-13),42.2 (C-14),27.0 (C-15),38.3 (C-16),36.3 (C-17),48.6 (C-18),37.1 (C-19),139.9 (C-20),118.0 (C-21),41.1 (C-22),34.1 (C-23),15.4 (C-24),18.3 (C-25),16.0 (C-26),14.7 (C-27),18.3 (C-28),26.2 (C-29),25.5 (C-30)。以上数据与文献报道基本一致[9],故鉴定化合物5为伪蒲公英甾醇。
化合物6:无色粉末,mp 213~215 ℃。1H-NMR (400 MHz,CDCl3) δ: 4.70 (2H,s,H-30),4.55 (1H,brs,H-3),2.04 (3H,s,3-CH3CO),0.92~2.48 (20H,m,H-1,2,6,7,11,12,15,16,21,22),1.08 (3H,d,J = 5.0 Hz,H-29),0.99 (3H,s,H-26),0.96 (3H,s,H-27),0.94 (3H,s,H-28),0.91 (3H,s,H-23),0.91 (3H,s,H-24),0.86 (3H,s,H-25);13C-NMR (100 MHz,CDCl3) δ: 38.6 (C-1),26.5 (C-2),81.3 (C-3),37.8 (C-4),55.3 (C-5),18.5 (C-6),34.3 (C-7),41.2 (C-8),50.7 (C-9),37.4 (C-10),21.7 (C-11),26.0 (C-12),39.5 (C-13),42.3 (C-14),26.7 (C-15),38.1 (C-16),34.8 (C-17),48.9 (C-18),39.4 (C-19),154.9 (C-20),25.7 (C-21),39.1 (C-22),28.2 (C-23),15.6 (C-24),16.8 (C-25),16.4 (C-26),15.0 (C-27),19.7 (C-28),25.2 (C-29),107.3 (C-30),171.3 (3-C = O),21.6 (3-CH3CO)。以上数据与文献报道基本一致[10],故鉴定化合物6为蒲公英甾醇乙酸酯。
化合物7:无色针状结晶(甲醇)。1H-NMR (400 MHz,CDCl3) δ: 5.34 (1H,d,J = 5.2 Hz,H-6),3.50 (1H,m,H-3),1.00 (3H,s,H-19),0.92 (3H,d,J = 8.0 Hz,H-21),0.86 (3H,t,J = 8.0 Hz,H-29),0.82 (3H,d,J = 7.6 Hz,H-26),0.80 (3H,d,J = 7.6 Hz,H-27),0.66 (3H,s,H-18);13C-NMR (100 MHz,CDCl3) δ: 141.2 (C-5),120.0 (C-6),69.5 (C-3),56.0 (C-17),55.4 (C-14),49.8 (C-9),45.1 (C-24),42.0 (C-13),42.0 (C-4),39.5 (C-12),37.2 (C-1),36.5 (C-20),36.7 (C-10),33.4 (C-22),31.7 (C-2),31.2 (C-7),31.6 (C-8),28.9 (C-25),28.5 (C-16),25.7 (C-23),24.2 (C-15),23.6 (C-28),21.7 (C-11),20.4 (C-26),19.3 (C-27),19.3 (C-19),19.0 (C-21),11.4 (C-18),11.3 (C-29)。以上数据与文献报道基本一致[11],故鉴定化合物7为β-谷甾醇。
化合物8:白色粉末。1H-NMR (400 MHz,Pyr-d5) δ: 3.94 (1H,m,H-3),5.36 (1H,d,J = 2.6 Hz,H-6),0.69 (3H,s,H-18),0.96 (3H,s,H-19),1.00 (3H,d,J = 6.1 Hz,H-21),0.88 (3H,d,J = 7.1 Hz,H-26),0.90 (3H,d,J = 7.1 Hz,H-27),0.91 (3H,t,J = 7.4 Hz,H-29),5.07 (1H,d,J = 7.7 Hz,H-1′),4.01 (1H,t,J = 7.8 Hz,H-2′),4.23 (1H,m,H-3′),4.23 (1H,m,H-4′),3.94 (1H,m,H-5′),4.42 (1H,d,J = 11.7 Hz,H-6′a),4.37 (1H,dd,J = 11.7,4.7 Hz,H-6′b);13C-NMR (100 MHz,Pyr-d5) δ: 141.4 (C-5),122.4 (C-6),103.1 (C-1′),79.1 (C-3′),79.0 (C-3),78.6 (C-5′),75.9 (C-2′),72.2 (C-4′),63.4 (C-6′),57.3 (C-14),56.8 (C-17),50.9 (C-9),46.6 (C-24),43.0 (C-13),40.5 (C-12),40.0 (C-4),38.0 (C-1),37.4 (C-10),36.9 (C-20),34.7 (C-22),32.7 (C-7),32.6 (C-8),30.8 (C-2),30.0 (C-25),29.3 (C-16),27.0 (C-23),25.0 (C-15),23.9 (C-28),21.8 (C-11),20.5 (C-27),19.9 (C-26),19.7 (C-19),19.5 (C-21),12.7 (C-29),12.5 (C-18)。以上数据与文献报道基本一致[12],故鉴定化合物8为β-胡萝卜苷。
化合物9:无色片状结晶(甲醇),mp 199~201 ℃。1H-NMR (400 MHz,DMSO-d6) δ: 7.33 (1H,d,J = 2.0 Hz,H-2),7.27 (1H,dd,J = 8.2,2.0 Hz,H-6),6.76 (1H,d,J = 8.2 Hz,H-5)。以上数据与文献报道基本一致[13],故鉴定化合物9为原儿茶酸。
化合物10:灰白色粉末。1H-NMR (400 MHz,DMSO-d6) δ: 11.02 (1H,s,NH-3),10.83 (1H,brs,1-NH),7.41 (1H,d,J = 5.7 Hz,H-6),5.45 (1H,d,J = 5.7 Hz,H-5);13C-NMR (100 MHz,DMSO-d6) δ: 164.0 (C-4),151.2 (C-2),141.9 (C-6),99.9 (C-5)。以上数据与文献报道基本一致[14],故鉴定化合物10为尿嘧啶。
化合物11:淡黄色无定形粉末,mp 255~257 ℃,FeCl3、Mg-HCl及Molish反应阳性,EI-MS m/z: 593 [M+H]+。1H-NMR (400 MHz,DMSO-d6) δ: 3.84 (3H,s,4′-OCH3),6.45 (1H,d,J = 2.2 Hz,H-6),6.79 (1H,d,J = 2.2 Hz,H-8),6.94 (1H,s,H-3),7.14 (2H,d,J = 9.0 Hz,H-3′,5′),8.05 (2H,d,J = 7.0 Hz,H-2′,6′),12.9 (1H,s,5-OH),1.07 (3H,d,J = 6.4 Hz,Rha-H-6),4.54 (1H,s,Rha-H-1),5.06 (1H,d,J = 7.3 Hz,Glc-H-1);13C-NMR (100 MHz,DMSO-d6) δ: 55.3 (4′-OCH3),94.5 (C-8),99.6 (C-6),104.3 (C-3),105.5 (C-10),114.4 (C-3′),114.4 (C-5′),122.8 (C-1′),128.2 (C-2′),128.2 (C-6′),156.6 (C-9),161.5 (C-5),162.2 (C-4′),162.6 (C-7),164.1 (C-2),182.3 (C-4),65.8 (Glc-C-6),69.3 (Glc-C-4),73.3 (Glc-C-2),75.4 (Glc-C-5),76.0 (Glc-C-3),99.8 (Glc-C-1),17.5 (Rha-C-6),68.5 (Rha-C-5),70.5 (Rha-C-2),71.2 (Rha-C-3),72.3 (Rha-C-4),100.4 (Rha-C-1)。以上数据与文献报道基本一致[15],故鉴定化合物11为蒙花苷。
化合物12:淡黄色粉末(甲醇),mp>300 ℃。1H-NMR (400 MHz,DMSO-d6) δ: 12.95 (1H,s,5-OH),10.57 (1H,brs,7-OH),10.57 (1H,brs,4′-OH),7.90 (1H,d,J = 8.8 Hz,H-2′),7.90 (1H,d,J = 8.8 Hz,H-6′),6.91 (1H,d,J = 8.8 Hz,H-3′),6.91 (1H,d,J = 8.8 Hz,H-5′),6.75 (1H,s,H-3),6.46 (1H,d,J = 2.0 Hz,H-8),6.17 (1H,d,J = 2.0 Hz,H-6);13C-NMR (100 MHz,DMSO-d6) δ: 164.3 (C-7),102.6 (C-3),181.5 (C-4),161.7 (C-5),98.8 (C-6),163.8 (C-2),94.2 (C-8),157.3 (C-9),103.5 (C-10),120.9 (C-1),128.2 (C-2′),128.2 (C-6′),116.2 (C-3′),116.2 (C-5′),160.8 (C-4′)。以上数据与文献报道基本一致[16],故鉴定化合物12为芹菜素。
化合物13:黄色细针晶(甲醇),mp 251~253 ℃。1H-NMR (400 MHz,DMSO-d6) δ: 0.81 (3H,d,J = 6.0 Hz,Rha-5-CH3),3.08~3.30 (1H,m,Rha-H-4),3.08~3.30 (1H,m,Rha-H-5),3.46~3.52 (1H,m,Rha-H-3),3.94~3.99 (1H,m,Rha-H-2),5.24 (1H,d,J = 1.7 Hz,Rha-H-1),6.20 (1H,d,J = 2.2 Hz,H-6),6.38 (1H,d,J = 2.2 Hz,H-8),6.87 (1H,d,J = 8.4 Hz,H-5′),7.26 (1H,dd,J = 8.7,2.0 Hz,H-6′),7.29 (1H,d,J = 2.0 Hz,H-2′),9.36,9.73,10.89 (各1H,brs,7,3′,4′-OH),12.66 (1H,s,5-OH);13C-NMR (100 MHz,DMSO-d6) δ: 17.8 (Rha-C-6),70.3 (Rha-C-5),70.6 (Rha-C-3),70.8 (Rha-C-2),71.5 (Rha-C-4),93.9 (C-8),99.0 (C-6),102.2 (Rha-C-1),104.4 (C-10),115.7 (C-2′),115.9 (C-5′),120.9 (C-6′),121.0 (C-1′),134.7 (C-3),145.7 (C-3′),148.9 (C-4′),156.9 (C-9),157.8 (C-2),161.8 (C-5),164.8 (C-7),178.3 (C-4)。以上数据与文献报道基本一致[17],故鉴定化合物13为槲皮苷。
化合物14:黄色针晶(甲醇),mp 259~262 ℃,盐酸-镁粉反应阳性,EI-MS m/z: 284 [M]+。H-NMR (400 MHz,DMSO-d6) δ: 12.96 (1H,s,5-OH),10.81 (1H,s,8-OH),7.94 (2H,d,J = 8.7 Hz,H-2′,6′),6.93 (2H,d,J = 8.8 Hz,H-3′,5′),6.90 (1H,d,J = 2.5 Hz,H-6),6.87 (1H,s,H-3),6.43 (1H,d,J = 2.1 Hz,H-8),3.88 (3H,s,4′-OCH3);13C-NMR (100 MHz,DMSO-d6) δ: 182.2 (C-4),164.6 (C-7),162.8 (C-2),161.9 (C-5),157.8 (C-9),128.9 (C-2′,6′),122.0 (C-1′),115.4 (C-3′,5′),105.3 (C-10),103.3 (C-3),99.3 (C-6),94.3 (C-8),55.4 (4′-OCH3)。以上数据与文献报道基本一致[16],故鉴定化合物14为金合欢素。
化合物15:淡黄色结晶(甲醇),mp 260~262 ℃,盐酸-镁粉和Molish反应均为阳性,酸水解检出葡萄糖。1H-NMR (400 MHz,DMSO-d6) δ: 12.92 (1H,s,5-OH),8.05 (2H,d,J = 8.9 Hz,H-2′,6′),7.14 (2H,d,J = 8.9 Hz,H-3′,5′),6.95 (1H,s,H-3),6.86 (1H,d,J = 1.8 Hz,H-8),6.46 (1H,d,J = 1.8 Hz,H-6),5.08 (1H,d,J = 7.4 Hz,Glc-H-1),3.87 (3H,s,4′-OCH3);13C-NMR (100 MHz,DMSO-d6) δ: 163.9 (C-2),103.9 (C-3),182.2 (C-4),161.6 (C-5),99.7 (C-6),163.2 (C-7),95.1 (C-8),157.1 (C-9),105.6 (C-10),55.73 (C-4′-OCH3),122.8 (C-1′),128.6 (C-2′),114.8 (C-3′),162.3 (C-4′),114.7 (C-5′),128.6 (C-6′),100.1 (Glc-C-1),73.3 (Glc-C-2),76.6 (Glc-C-3),69.7 (Glc-C-4),76.3 (Glc-C-5),60.8 (Glc-C-6)。以上数据与文献报道基本一致[18],故鉴定化合物15为金合欢素-7-O-β-D-葡萄糖苷。
化合物16:淡黄色针状结晶(甲醇),mp 194 ℃。1H-NMR (400 MHz,CD3OD) δ: 7.32 (2H,s,H-2,6),3.87 (6H,s,3,5-OCH3);13C-NMR (100 MHz,CD3OD) δ: 170.0 (C-7),149.0 (C-3,5),142.0 (C-4),122.1 (C-1),108.7 (C-2,6),59.9 (3,5-OCH3)。以上数据与文献报道基本一致[19],故鉴定化合物16为4-羟基-3,5-二甲氧基苯甲酸。
| [1] | 中国科学院中国植物志编辑委员会. 中国植物志[M]. 北京: 科学出版社, 1979 . |
| [2] | 詹亚华. 中国神农架中药资源[M]. 武汉: 湖北科学技术出版社, 1994 . |
| [3] | 植飞, 孔令飞, 彭司勋. 中药大蓟的化学及药理研究进展[J]. 中草药 , 2001, 31 (7) :664–667. |
| [4] | 张悦, 阮汉利, 张勇慧, 等. 鄂西大蓟根的化学成分的研究[J]. 天然产物研究与开发 , 2010, 22 (1) :58–59. |
| [5] | 张悦, 阮汉利, 张勇慧, 等. 鄂西大蓟化学成分的研究[J]. 医药导报 , 2007, 26 (12) :1425–1426. |
| [6] | Yang N Y, Wang L Y, Zhang Y W. Immunological activities of components from leaves of Liriodendron chinensis[J]. Chin Herb Med , 2015, 7 (3) :279–282. |
| [7] | Morandat S, Bortolato M, Anker G, et al. Plasmalogens protect unsaturated lipids against UV-induced oxidation in monolayer[J]. Biochim Biophys Acta , 2003, 1616 (2) :137–146. |
| [8] | 马春辉, 李伯刚, 徐庆, 等. 柳叶忍冬的化学成分研究[J]. 应用与环境生物学报 , 2006, 12 (4) :487–495. |
| [9] | Ma X M, Di D L, Shi Y P. Triterpenoids and steroids from Ixeridium gracile[J]. Chem Nat Compd , 2008, 44 (3) :399–401. |
| [10] | 滑艳, 何荔, 汪汉卿. 白茎绢蒿化学成分的研究[J]. 天然产物研究与开发 , 2003, 15 (3) :219–221. |
| [11] | 李伟, 时圣明, 唐云, 等. 合子草化学成分的研究(I)[J]. 中草药 , 2014, 45 (15) :2143–2147. |
| [12] | Chadwick L R, Nikolic D, Burdette J E, et al. Estrogens and congeners from Spent hops (Humulus lupulus)[J]. J Nat Prod , 2004, 67 (12) :2024–2032. |
| [13] | 马趣环, 石晓峰, 范彬, 等. 糙叶败酱正丁醇部位化学成分研究[J]. 中草药 , 2015, 46 (11) :1593–1596. |
| [14] | Ding Z G, Zhao J Y, Yang P W. 1H and 13C NMR assignments of eight nitrogen containing compounds from Nocardia alba sp. nov (YIM 30243T)[J]. Magn Reson Chem , 2009, 47 (4) :366–370. |
| [15] | Quintin J, Lewin G. Semisynthesis of linarin, acacetin, and 6-Iodoapigenin derivatives from diosmin[J]. J Nat Prod , 2004, 67 (9) :1624–1627. |
| [16] | Miyazawa M, Hisama M. Antimutagenic activity of flavonoids from Chrysanthemum morifolium[J]. Biosci Biotech Biochem , 2003, 67 (10) :2091–2099. |
| [17] | Pyo M K, Lee Y Y, Yun-Choi H S. Anti-platelet effect of the phenolic constituents isolated from the leaves of Magnolia obovata[J]. Nat Prod Sci , 2002, 8 (4) :147–151. |
| [18] | Li Y L, Li J, Wang N L, et al. Flavonoids and a new polyacetylene from Bidens parviflora Willd[J]. Molecules , 2008, 13 (8) :1931–1941. |
| [19] | 李药兰, 苏妙贤, 岑颖洲, 等. 小紫金牛的化学成分研究[J]. 中药材 , 2006, 29 (4) :331–333. |
 2016, Vol. 47
2016, Vol. 47


