黑水缬草Valeriana amurensis Smir. ex Kom. 为败酱科(Valerianaceae)缬草属Valeriana L. 多年生草本植物,黑水缬草药材(干燥根及根茎)味辛、甘,性温,具有安神、理气和止痛的功效,归心、肝经;可用于失眠、癔症及癫痫等神经系统疾病的治疗。几个世纪以来,缬草及其他一些缬草属植物在欧美一直用于治疗失眠[1-3],现代研究表明,缬草属植物具有抗焦虑、抗抑郁、解痉、镇静、抗肿瘤及抗艾滋病毒等方面的药理作用[4-8]。黑水缬草主要分布在我国东北部,尤其在大兴安岭地区资源丰富。本课题组前期研究首次发现黑水缬草具有显著的抗阿尔茨海默症(AD)作用,并进一步确定了抗AD的有效部位为黑水缬草提取物的大孔吸附树脂柱色谱50%乙醇洗脱组分,该有效部位在体外实验中对PC12细胞损伤模型亦具有很好的保护作用[9],基于此,本实验继续对黑水缬草抗AD的有效部位进行了系统的活性成分研究,以进一步明确黑水缬草发挥神经保护作用的药效物质基础。分离得到的11个化合物分别鉴定为 (+)-梣皮树脂醇-4,4′-二-O-β-D-双吡喃葡萄糖苷 [(+)-medioresinol-4,4′-di-O-β-D- glucopyranoside,1]、(+)-紫丁香-4,4′-二-O-β-D-吡喃葡萄糖苷 [(+)-syringaresinol-4,4′-di-O-β-D-glucopyranoside,2]、青刺尖木脂醇苷(prinsepiol-4-O-β-D-glucopyranoside,3)、(+)-8,8′-二羟基-松脂素-4,4′-二-O-β-D-吡喃葡萄糖苷 [(+)-8,8′-dihydroxy-pinoresinol-4,4'-di-O-β-D-glucopy- ranoside,4]、青刺尖木脂醇(prinsepiol,5)、jatamanin A(6)、7-羟基-8-(羟甲基)-4-亚甲基六氢环戊烯[并]吡喃-1(3H)-酮(7-hydroxy-8-(hydroxymethyl)-4- methylenehexahydrocyclopenta [c] pyran-1(3H)-one,7)、4-羟甲基-环戊烯[并]吡喃-7-甲醛(4-hydroxymethyl-cyclopenta [c] pyran-7-carboxaldehyde,8)、patriscabroside III(9)、jatamanin E(10)及败酱苷(patrinoside,11)。其中环烯醚萜类化合物6~10为该植物中首次分离得到,双环氧木脂素类化合物均能明显减轻Aβ1-42所致的PC12细胞损伤,为黑水缬草抗AD有效部位中发挥神经保护作用的药效物质基础。
1 仪器与材料Bruker-400超导核磁共振光谱仪(Bruker公司);Waters Xero Q-TOF型ESI-MS(Waters公司);2535-2998-2414制备型HPLC(Waters公司);硅胶(青岛海洋化工厂,200~300目);ODS-AM(YMC公司);AB-8大孔吸附树脂(南开大学化工厂)。CO2细胞培养箱(TC 2323,美国SHELDON);酶标仪(VICTORTM×3,PerkinElmer,Inc.,美国);DMEM完全培养液(NRH0020,海克隆生物化学制品有限公司);胎牛血清(海克隆生物化学制品有限公司);胰蛋白酶(LOT1379361);DMEM冻存液(Exporation 06/2008 0231 XIASI BIO);双抗(青-链霉素溶液,北京赛驰生物科技有限公司);Aβ1-42(北京博奥森生物技术有限公司);维生素E(VE,H20073294,国药控股星鲨制药有限公司)。
实验原药材于2010年8月采于黑龙江省大兴安岭地区呼玛县,经黑龙江中医药大学药学院中药鉴定教研室苏连杰教授鉴定为败酱科缬草属植物黑水缬草Valeriana amurensis Smir. ex Kom.。PC12细胞株购于中国科学院上海细胞所。
2 方法 2.1 黑水缬草抗AD有效部位的制备将干燥黑水缬草根及根茎45 kg,以75%乙醇回流提取,得到总提取物8 211.96 g,将该提取物以水混悬,以石油醚萃取,将水层减压回收溶剂,得到水层萃取物6 144.60 g,取其中2.30 kg经AB-8型大孔吸附树脂柱色谱,以水及50%、95%乙醇依次洗脱,减压回收各个洗脱组分,得到50%乙醇洗脱组分521.13 g,该组分即为黑水缬草抗AD有效部位。
2.2 化合物的分离取黑水缬草抗AD有效部位80.0 g,经二氯甲烷-甲醇(15∶1、8∶1、4∶1、2∶1)梯度洗脱,共得7个组分(Fr. 1~7),Fr. 1(15.84 g)经正相硅胶柱色谱分离,二氯甲烷-甲醇(40∶1)反复洗脱得到化合物7(51 mg)和8(46 mg),Fr. 3(10.45 g)经反相ODS柱色谱分离,甲醇-水(25%)洗脱,并经制备型反相HPLC乙腈-水(15%)洗脱分离得到化合物5(33 mg)、6(28 mg)、10(32 mg)和11(43 mg),Fr. 5(28.58 g)经反相ODS柱色谱,甲醇-水(15%)洗脱,并经制备型反相HPLC,乙腈-水(7%)洗脱分离得到化合物1(35 mg)、2(37 mg)、3(52 mg)、4(39 mg)和9(40 mg)。
2.3 单体化合物神经细胞保护活性筛选采用MTT法对化合物1~11的神经保护活性进行筛选[10],在DMEM培养基中加入10%的胎牛血清,并加入1%双抗(100 U/mL链霉素,100 U/mL青霉素),使用该培养液将PC12细胞放在37 ℃、5% CO2、饱和湿度培养箱中培养,经胰酶消化传代后,选取对数生长期细胞用于实验。实验共分14组,每组8孔。空白对照组、模型组每孔加入无血清DMEM低糖培养基120 μL;各给药组每孔加入无血清DMEM低糖培养基配制的各浓度(25、12.5和5 μmol/L)的单体化合物及各浓度(50、25和12.5 μmol/L)的维生素E(VE)120 μL;37 ℃培养4 h后除空白对照组加10 μL的无血清DMEM低糖培养基外,其他各组均加入10 μL Aβ1-42并使其终浓度为1.5 μmol/L;37 ℃培养24 h后,每孔加入5 mg/mL MTT 20 μL,继续培养4 h后吸去DMEM培养基,每孔加入DMSO 150 μL,振摇混匀10 min,待孔内颗粒完全溶解后,在酶标仪492 nm处测定吸光度(A)值。上述实验重复3次,结果以x±s表示,各组细胞活力=各组细胞A值/空白对照组细胞A值。
3 结果与分析 3.1 结构鉴定化合物1:白色无定形粉末,可溶于甲醇、水,难溶于二氯甲烷;Molish反应呈阳性;ESI-MS m/z: 735 [M+Na]+。1H-NMR (400 MHz,DMSO-d6) δ: 6.95 (1H,d,J = 2.0 Hz,H-2),7.05 (1H,d,J = 8.4 Hz,H-5),6.86 (1H,dd,J = 2.0,8.4 Hz,H-6),5.16 (1H,d,J = 4.2 Hz,H-7),3.25 (1H,m,H-8),3.82 (1H,dd,J = 2.8,8.1 Hz,H-9a),4.17 (1H,m,H-9b),6.66 (2H,s,H-2′,6′),5.03 (1H,d,J = 3.6 Hz,H-7′),3.25 (1H,m,H-8′),3.82 (1H,dd,J = 2.8,8.1 Hz,H-9′a),4.17 (1H,m,H-9′b),3.76 (3H,s,3-OCH3),3.77 (6H,s,3′,5′-OCH3),4.88 (2H,d,J = 6.0 Hz,glu-H-1″,1′′′);13C-NMR (100 MHz,DMSO-d6) δ: 135.2 (C-1),110.6 (C-2),145.9 (C-3),149.0 (C-4),115.3 (C-5),118.2 (C-6),84.9 (C-7),53.7 (C-8),71.3 (C-9),133.8 (C-1′),104.2 (C-2′,6′),152.7 (C-3′,5′),137.2 (C-4′),85.1 (C-7′),53.6 (C-8′),71.2 (C-9′),55.7 (3-OCH3),55.7 (3′,5′-OCH3);δglu-C (1″-6″): 100.2,73.2,76.5,69.7,77.0,60.7;δglu-C (1′′′-6′′′): 102.7,74.2,76.9,70.0,77.2,60.9。以上数据与文献报道一致[11],故鉴定化合物1为(+)-梣皮树脂醇-4,4′-二-O-β-D-双吡喃葡萄糖苷。
化合物2:白色无定形粉末,可溶于甲醇、水,难溶于二氯甲烷;Molish反应呈阳性;ESI-MS m/z: 765 [M+Na]+。1H-NMR (400 MHz,DMSO-d6) δ: 6.66 (4H,s,H-2,6,2′,6′),4.67 (2H,d,J = 3.0 Hz,H-7,7′),3.03 (2H,m,H-8,8′),3.82 (2H,d,J = 9.0 Hz,H-9a,9′a),4.20 (2H,m,H-9b,9′b),3.75 (12H,s,3,3′,5,5′-OCH3),4.89 (2H,d,J = 6.1 Hz,glu-H-1″,1′′′);13C-NMR (100 MHz,DMSO-d6) δ: 133.9 (C-1,1′),104.4 (C-2,2′),152.8 (C-3,3′),137.3 (C-4,4′),152.8 (C-5,5′),104.4 (C-6,6′),85.3 (C-7,7′),53.8 (C-8,8′),71.6 (C-9,9′),56.6 (3,3′,5,5′-OCH3);δglu-C (1″-6″,1′′′-6′′′): 102.9,74.4,77.4,70.1,76.7,61.1。以上数据与文献报道一致[11],故鉴定化合物2为 (+)-紫丁香-4,4′-二-O-β-D-吡喃葡萄糖苷。
化合物3:白色无定形粉末,可溶于甲醇、水,难溶于二氯甲烷;Molish反应呈阳性;ESI-MS m/z: 575 [M+Na]+。1H-NMR (400 MHz,CD3OD) δ: 7.01 (1H,d,J = 2.0 Hz,H-2),7.02 (1H,d,J = 8.0 Hz,H-5),6.92 (1H,dd,J = 2.0,8.0 Hz,H-6),4.86 (1H,s,H-7),3.87 (1H,d,J = 9.5 Hz,H-9a),3.96 (1H,d,J = 9.5 Hz,H-9b),6.98 (1H,d,J = 2.0 Hz,H-2′),6.70 (1H,d,J = 8.0 Hz,H-5′),6.72 (1H,dd,J = 2.0,8.0 Hz,H-6′),4.83 (1H,s,H-7′),3.87 (1H,d,J = 9.5 Hz,H-9′a),3.96 (1H,d,J = 9.5 Hz,H-9′b),3.70 (6H,s,3,3′-OCH3),4.81 (1H,d,J = 8.5 Hz,glu-H-1″);13C-NMR (100 MHz,CD3OD) δ: 133.0 (C-1),113.5 (C-2),150.2 (C-3),147.5 (C-4),117.2 (C-5),121.5 (C-6),88.7 (C-7),89.3 (C-8),76.8 (C-9),129.6 (C-1′),112.9 (C-2′),148.7 (C-3′),147.4 (C-4′),115.9 (C-5′),121.7 (C-6′),89.0 (C-7′),89.4 (C-8′),76.8 (C-9′),56.9 (3-OCH3),56.7 (3′-OCH3);δglu-C (1″-6″): 102.6,74.9,77.7,71.3,78.0,62.6。以上数据与文献报道一致[12],故鉴定化合物3为青刺尖木脂醇苷。
化合物4:白色无定形粉末,可溶于甲醇、水,难溶于二氯甲烷;Molish反应呈阳性;ESI-MS m/z: 737 [M+Na]+。1H-NMR (400 MHz,CD3OD) δ: 7.01 (2H,d,J = 1.7 Hz,H-2,2′),7.03 (2H,d,J = 8.5 Hz,H-5,5′),6.86 (2H,dd,J = 1.7,8.5 Hz,H-6,6′),4.93 (2H,s,H-7,7′),4.04 (2H,d,J = 9.2 Hz,H-9a,9′a),3.84 (2H,d,J = 9.2 Hz,H-9b,9′b),3.76 (6H,s,3,3′-OCH3),4.87 (2H,d,J = 7.4 Hz,glu-H-1″,1′′′);13C-NMR (100 MHz,CD3OD) δ: 131.5 (C-1,1′),112.5 (C-2,2′),148.4 (C-3,3′),146.0 (C-4,4′),114.7 (C-5,5′),119.8 (C-6,6′),87.7 (C-7,7′),86.8 (C-8,8′),73.3 (C-9,9′),55.8 (3,3′-OCH3);δglu-C (1″-6″,1′′′-6′′′): 100.3,74.6,77.0,69.7,76.9,60.7。以上数据与文献报道一致[13],故鉴定化合物4为 (+)-8,8′-二羟基-松脂素-4,4′-二-O-β-D-吡喃葡萄糖苷。
化合物5:无色簇状结晶(甲醇),易溶于甲醇;ESI-MS m/z: 409 [M+Na]+。1H-NMR (400 MHz,CD3OD) δ: 7.06 (2H,brs,H-2,2′),6.81 (2H,d,J = 8.0 Hz,H-5,5′),6.87 (2H,brd,J = 8.0 Hz,H-6,6′),4.98 (2H,s,H-7,7′),4.00 (2H,d,J = 9.4 Hz,H-9a,9′a),4.12 (2H,d,J = 9.4 Hz,H-9b,9′b),3.88 (6H,s,3,3′-OCH3);13C-NMR (100 MHz,CD3OD) δ: 129.8 (C-1,1′),113.1 (C-2,2′),148.9 (C-3,3′),147.7 (C-4,4′),115.9 (C-5,5′),121.8 (C-6,6′),89.2 (C-7,7′),89.3 (C-8,8′),77.0 (C-9,9′),56.7 (3,3′-OCH3)。以上数据与文献报道一致[14],故鉴定化合物5为青刺尖木脂醇。
化合物6:无色针晶(甲醇),易溶于甲醇;ESI-MS m/z: 199 [M+H]+。1H-NMR (400 MHz,CD3OD) δ: 4.32 (1H,d,J = 11.5 Hz,H-3a),4.97 (1H,d,J = 10.8 Hz,H-3b),3.22 (1H,m,H-5),1.88 (1H,t,J = 10.9 Hz,H-6a),2.07 (1H,t,J = 11.5 Hz,H-6b),3.66 (1H,brs,H-7),2.89 (1H,d,J = 10.8 Hz,H-9),1.37 (3H,s,H-10),4.95 (1H,s,H-11a),5.00 (1H,s,H-11b);13C-NMR (100 MHz,CD3OD) δ: 175.1 (C-1),71.2 (C-3),144.2 (C-4),41.1 (C-5),40.8 (C-6),81.5 (C-7),86.3 (C-8),53.7 (C-9),22.2 (C-10),113.9 (C-11)。以上数据与文献报道一致[15],故鉴定化合物6为jatamanin A。
化合物7:无色针晶(甲醇),易溶于甲醇;ESI-MS m/z: 199 [M+H]+。1H-NMR (400 MHz,CD3OD) δ: 4.29 (1H,brs,H-3),3.37 (1H,m,H-5),1.56 (1H,m,H-6a),2.05 (1H,dd,J = 13.2,7.4 Hz,H-6b),4.62 (2H,m,H-7),2.31 (1H,m,H-8),2.91 (1H,t,J = 10.0 Hz,H-9),3.77 (1H,dd,J = 4.8,10.8 Hz,H-10a),3.70 (1H,t,J = 8.1 Hz,H-10b),4.96 (1H,s,H-11a),5.09 (1H,s,H-11b);13C-NMR (100 MHz,CD3OD) δ: 177.5 (C-1),73.7 (C-3),144.3 (C-4),41.1 (C-5),41.5 (C-6),72.6 (C-7),52.2 (C-8),45.1 (C-9),62.1 (C-10),113.9 (C-11)。以上数据与文献报道一致[16],故鉴定化合物7为7-羟基-8-(羟甲基)-4-亚甲基六氢环戊烯[并]吡喃-1(3H)-酮。
化合物8:黄色针状结晶(甲醇),易溶于甲醇;ESI-MS m/z: 177 [M+H]+。1H-NMR (400 MHz,CD3OD) δ: 9.20 (1H,d,J = 7.6 Hz,H-1),8.09 (1H,d,J = 7.4 Hz,H-3),6.70 (1H,d,J = 4.6 Hz,H-6),7.97 (1H,d,J = 4.4 Hz,H-7),9.82 (1H,d,J = 7.6 Hz,H-10),4.85 (2H,s,H-11);13C-NMR (100 MHz,CD3OD) δ: 151.9 (C-1),142.9 (C-3),125.8 (C-4),136.5 (C-5),110.4 (C-6),148.0 (C-7),125.3 (C-8),124.2 (C-9),186.4 (C-10),59.5 (C-11)。以上数据与文献报道一致[17],故鉴定化合物8为4-羟甲基-环戊烯[并]吡喃-7-甲醛。
化合物9:白色无定形粉末,可溶于甲醇、水,难溶于二氯甲烷;ESI-MS m/z: 495 [M+H]+。1H-NMR (400 MHz,CD3OD) δ: 4.31 (1H,dd,J = 11.4,6.7 Hz,H-1a),4.22 (1H,dd,J = 11.4,6.7 Hz,H-1b),2.46 (1H,m,H-4),2.03 (1H,m,H-5),1.86 (1H,m,H-6a),2.05 (1H,m,H-6b),3.91 (1H,t,J = 4.7 Hz,H-7),2.31 (1H,m,H-9),1.25 (3H,s,H-10),1.04 (3H,d,J = 6.4 Hz,H-11),4.29 (1H,d,J = 7.7 Hz,glu-H-1′),4.92 (1H,d,J = 2.0 Hz,api-H-1″);13C-NMR (100 MHz,CD3OD) δ: 67.6 (C-1),179.5 (C-3),40.1 (C-4),40.6 (C-5),37.6 (C-6),89.0 (C-7),81.7 (C-8),46.4 (C-9),22.9 (C-10),14.4 (C-11);δglu-C: 105.1,75.5,78.2,71.7,77.0,68.6;δapi-C: 110.0,78.2,80.7,75.2,65.9。以上数据与文献报道一致[18],故鉴定化合物9为patriscabroside Ⅲ。
化合物10:无色簇状结晶(甲醇),易溶于甲醇;ESI-MS m/z: 215 [M+H]+。1H-NMR (400 MHz,CD3OD) δ: 5.63 (1H,brs,H-3),1.99 (1H,m,H-4),2.24 (1H,m,H-5),2.36 (1H,dd,J = 7.2,13.7 Hz,H-6a),1.80 (1H,m,H-6b),4.00 (1H,m,H-7),2.75 (1H,d,J = 4.0 Hz,H-9),1.29 (3H,s,H-10),3.38 (2H,brs,H-11);13C-NMR (100 MHz,CD3OD) δ: 173.9 (C-1),98.7 (C-3),49.9 (C-4),35.7 (C-5),43.4 (C-6),78.8 (C-7),87.7 (C-8),51.5 (C-9),20.4 (C-10),62.6 (C-11)。以上数据与文献报道一致[19],故鉴定化合物10为jatamanin E。
化合物11:白色无定形粉末,可溶于甲醇,难溶于二氯甲烷;Molish反应阳性;ESI-MS m/z: 485 [M+Na]+。1H-NMR (400 MHz,CD3OD) δ: 5.90 (1H,d,J = 5.2 Hz,H-1),6.36 (1H,s,H-3),3.01 (1H,m,H-5),1.83 (1H,ddd,J = 4.8,8.4,13.0 Hz,H-6a),2.04 (1H,m,H-6b),4.31 (1H,m,H-7),2.15 (1H,ddd,J = 4.8,8.4,12.8 Hz,H-8),3.18 (1H,m,H-9),3.83 (1H,m,H-10a),3.75 (1H,m,H-10b),4.27 (1H,d,J = 12.6 Hz,H-11a),4.08 (1H,d,J = 12.6 Hz,H-11b);δisovaleryl-H: 2.06 (2H,m,H-2′),1.94 (1H,m,H-3′),0.96 (3H,d,J = 6.6 Hz,H-4′),0.96 (3H,d,J = 6.6 Hz,H-5′);4.28 (1H,d,J = 8.0 Hz,glu-H-1″);13C-NMR (100 MHz,CD3OD) δ: 93.6 (C-1),140.1 (C-3),116.4 (C-4),34.1 (C-5),40.9 (C-6),73.3 (C-7),49.0 (C-8),42.7 (C-9),62.3 (C-10),69.7 (C-11);δisovaleryl-C: 173.3 (C-1′),44.2 (C-2′),26.8 (C-3′),22.6 (C-4′),22.6(C-5′);δglu-C (1″-6″): 103.4,75.1,78.1,71.7,78.0,62.8。以上数据与文献报道一致[20],故鉴定化合物11为败酱苷。
3.2 对Aβ1-42所致PC12细胞损伤的保护作用不同浓度的单体化合物对Aβ1-42所致PC12细胞损伤的保护作用见图 1,其中双环氧木脂素类化合物均对Aβ1-42所致PC12细胞损伤具有很好的保护作用,除化合物5外,相同浓度条件下,其活性优于阳性对照药物VE。其中25和12.5 μmol/L浓度的化合物1~4及25 μmol/L浓度的化合物5与模型组比较,其PC12细胞活力具有显著性差异(P<0.01);而5 μmol/L浓度的化合物2和4及12.5 μmol/L浓度的化合物5与模型组比较,其PC12细胞活力具有显著性差异(P<0.05)。
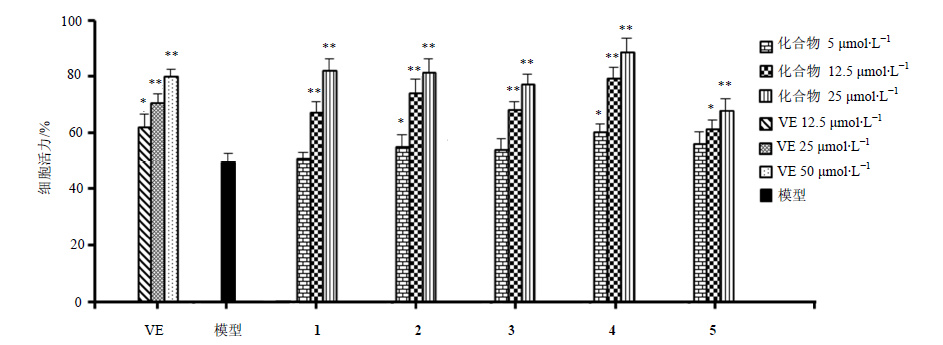
|
图 1 化合物1~5 对Aβ1-42 致PC12 细胞损伤的影响 Fig.1 Effects of tested compounds on Aβ1-42-induced PC12 cells injury |
环烯醚萜类化合物6~11对Aβ1-42所致PC12细胞损伤无明显保护作用,与模型组比较差异不显著(P>0.05)。综上结果表明,双环氧木脂素类化合物为黑水缬草神经保护作用的药效物质基础。
| [1] | Houghton P J. The biological activity of Valerian and related plants[J]. J Ethnopharmacol, 1988, 22 (2) :121–142 . |
| [2] | Houghton P J. The scientific basis for the reputed activity of Valerian[J]. J Pharm Pharmacol, 1999, 51 (5) :505–512 . |
| [3] | Yager J, Siegfreid S L, DiMattero T L. Use of alternative remedies by psychiatric patients: Illustrative vignettes and a discussion of the issues[J]. Am J Psychiatry, 1999, 156 (9) :1432–1438 . |
| [4] | Bounthanh C, Bergmann C, Beck J P, et al. Valepotrictes, a new class of cytotoxic and antitumor agents[J]. Planta Med, 1981, 41 (1) :21–28 . |
| [5] | Tortarolo M, Braun R, Hübner G E, et al. In vitro effects of epoxide-bearing alepotriates on mouse early hematopoietec progenitor cells and human T-lymphocytes[J]. Arch Toxicol, 1982, 51 (1) :37–42 . |
| [6] | Morazzoni P, Bombardelli E. Valeriana officinalis: Traditional use and recent evaluation of activity[J]. Fitoterapia, 1995, 66 (2) :99–112 . |
| [7] | Murakami N, Ye Y, Kawanishi M, et al. New rev-transport inhibitor with anti-HIV activity from Valerianae Radix[J]. Bioorg Med Chem Lett, 2002, 12 (20) :2807–2810 . |
| [8] | Hattesohl M, Feistel B, Sievers H, et al. Extracts of Valeriana officinalis L. s. l. show anxiolytic and antidepressant effects but neither sedative nor myorelaxant properties[J]. Phytomedicine, 2008, 15 (1/2) :2–15 . |
| [9] | 左月明. 黑水缬草抗老年痴呆有效部位化学成分和药理作用研究[D]. 哈尔滨: 黑龙江中医药大学, 2007. |
| [10] | Wang C F, Xiao Y, Yang B Y, et al. Isolation and screened neuroprotective active constituents from the Roots and Rhizomes of Valeriana amurensis[J]. Fitoterapia, 2014, 96 :48–55 . |
| [11] | Zheng L P, He Z G, Wu Z J, et al. Chemical constituents from Dendropanax dentiger[J]. Chem Nat Compd, 2012, 48 (5) :883–885 . |
| [12] | Piccinelli A L, Arana S, Caceres A, et al. New Lignans from the roots of Valeriana prionophylla with antioxidative and vasorelaxant activities[J]. J Nat Prod, 2004, 67 (7) :1135–1140 . |
| [13] | 于德泉, 杨峻山. 分析化学手册(第七分册), 核磁共振波谱分析[M].第2版. 北京: 化学工业出版社出版, 2002 . |
| [14] | Xu Y Q, Zhao B, Yang L X. (1S,3aR,4S,6aR)-1-(4-Hydroxy-3-methoxyphenyl)-4-(4-hydroxy-3-methylphenyl) perhydrofuro[3,4-c] furan-3a,6a-diol hexahydrate[J]. Acta Crystallographica Section E: Structure Reports Online, 2005, 61 (8) :o2559–o2560 . |
| [15] | Lin S, Chen T, Liu X H, et al. Iridoids and lignans from Valeriana jatamansi[J]. J Nat Prod, 2010, 73 (4) :632–638 . |
| [16] | Zhang Y, Lu Y, Zhang L, et al. Terpenoids from the roots and rhizomes of Nardostachys chinensis[J]. J Nat Prod, 2005, 68 (7) :1131–1133 . |
| [17] | Chen Y G, Yu L L, Huang R, et al. 11-Methoxyviburtinal, a new iridoid from Valeriana jatamansi[J]. Arch Pharm Res, 2005, 28 (10) :1161–1163 . |
| [18] | Kouno I, Yasuda I, Mizoshiri H, et al. Two new iridolactones and their glycosides from the roots of Patrinia scabra[J]. Phytochemistry, 1994, 37 (2) :467–472 . |
| [19] | Dinda B, Debnath S, Banik R. Naturally occurring iridoids and secoiridoids. An updated review, part 4[J]. Chem Pharm Bull, 2011, 59 (7) :803. |
| [20] | Tomassini L, Brkic D, Foddai S, et al. Iridoid glucosides from Viburnum rhytidophyllum[J]. Phytochemistry, 1997, 44 (4) :751–753 . |
 2016, Vol. 47
2016, Vol. 47


