贡山三尖杉Cephalotaxus lanceolata K. M. Feng是三尖杉科(Cephalotaxaceae)三尖杉属Cephalotaxus Sieb. et Zucc.ex Endl. 植物,其果实入药有润肺、止咳、消积之效[1]。国内外对该属植物的研究主要集中在生物碱[2, 3, 4, 5, 6, 7, 8]、黄酮[9]、二萜[10, 11, 12]等成分,而对其倍半萜类成分报道很少;对贡山三尖杉的研究也很少[13]。为了寻找更多结构新颖的活性成分,本课题组对产自云南省贡山县高黎贡山的贡山三尖杉枝叶95%乙醇提取物进行系统化学成分研究,从中分离得到11个倍半萜类化合物(图 1),分别鉴定为贡山三尖杉倍半萜A(lanceoloside A,1)、贡山三尖杉倍半萜B(lanceoloside B,2)、贡山三尖杉倍半萜C(lanceoloside C,3)、9-hydroxy-4,7-megastigmadien- 3-one(4)、corchoionol C(5)、9,10-dihydroxy-4,7- megastigmadien-3-one(6)、5,12-epoxy-9-hydroxy-7- megastigmen-3-one(7)、5,12-epoxy-6,9-hydroxy-7- megastigmen-3-one(8)、loliolide(9)、(3S,5R,8S)-5,8- epoxy-6-megastigmadien-3,9-diol(10)、clovandiol(11)。化合物1为未见文献报道的新化合物,化合物2~3为2个新天然产物,化合物4~11均为首次从该种植物中分离得到。
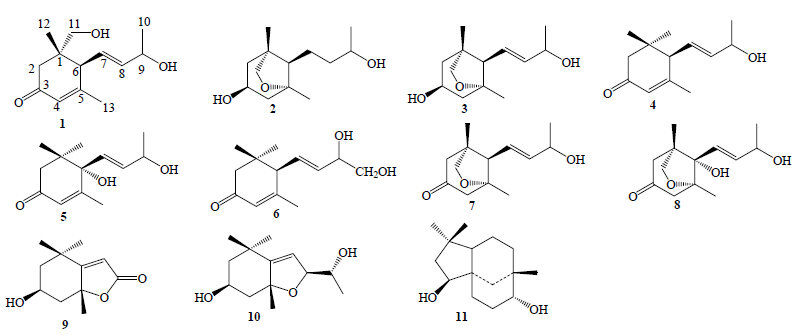 | 图 1 化合物1~11的结构Fig.1 Structures of compounds 1—11 |
薄层色谱硅胶GF254(烟台江友硅胶开发有限公司)、柱色谱硅胶(200~300目);Sephadex LH-20(40~70 μm)填料(美国GE公司),ODS填料(日本YMC公司);薄层色谱制备板(烟台江友硅胶开发有限公司);AVANCE-400、500 MHz核磁共振波谱仪(瑞士Bruker公司);Agilent LC/MSD和Agilent Q-TOF高分辨质谱仪(美国Agilent公司);Bruker FTIR Vector 22红外光谱仪(瑞士Bruker公司);Shimadzu UV-2550分光光度计(日本Shimadzu公司);Perkin-Elmer 341数字旋光仪(美国Perkin- Elmer公司);Buchi Sepacore C-615中压制备色谱系统(瑞士Buchi公司)。
贡山三尖杉全草于2010年8月采自云南省怒江州贡山县高黎贡山,经云南省怒江州民族医药研究所周元川所长鉴定为贡山三尖杉Cephalotaxus lanceolata K. M. Feng,植物标本(201008070)现保存在第二军医大学药学院天然药化教研室标本室。
2 提取与分离贡山三尖杉干燥枝叶9.3 kg,粉碎,用95%乙醇冷浸提取3次,每次48 h,提取液浓缩后以2%~3% HCl调节pH至2~3,滤过,滤液用CHCl3萃取除去非生物碱成分后水层用饱和Na2CO3调节pH至10,CHCl3萃取得到总生物碱9 g,不溶于HCl的滤渣和CHCl3萃取过的水层分别用石油醚、醋酸乙酯萃取,减压回收得醋酸乙酯部位135 g;将醋酸乙酯部位经常压硅胶柱色谱分离,石油醚-醋酸乙酯(100∶1、50∶1、30∶1、10∶1、5∶1、1∶1)梯度洗脱,得到8个流分Fr. 1~8。将Fr. 3(7.2 g)依次经过Sephadex LH-20(甲醇)及硅胶柱色谱分离,得到化合物1(15 mg)、4(6 mg)、6(21 mg)9(3 mg);Fr. 5(12.8 g)经过反相中压制备色谱,甲醇-水(40%~100%)梯度洗脱,得到6个部分Fr. 5-1~5-6。将Fr. 5-2(140 mg)经过Sephadex LH-20(甲醇)及硅胶柱色谱分离纯化,得到化合物2(7 mg)、3(9 mg)、7(15 mg)和8(7 mg);将Fr. 5-5(270 mg)依次经过Sephadex LH-20(甲醇)及硅胶柱色谱分离纯化,得到化合物5(30 mg)、10(5 mg)、11(13 mg)。
3 结构鉴定化合物1:白色无定形粉末,[α]D20 +117.8°(c 0.09, MeOH);IRv manKBr(cm−1): 3 376, 2 931, 1 639, 1 076, 1 037。高分辨质谱HR-ESI-MS给出准分子离子峰 [M+H]+ m/z: 225.141 2(计算值225.141 7),结合1H-NMR和13C-NMR,确定分子式为C13H20O3,不饱和度为4。根据1H-NMR、13C-NMR和DEPT谱(表 1)中的信息,可以判断该化合物含有3个季碳,其中2个sp2季碳 (δC 201.8 s, 165.9 s) 和1个sp3季碳 (δC 42.3 s);5个次甲基,其中1个连氧的次甲基 (δH 4.29 m, δC 68.9 d);2个亚甲基,其中有1个连氧的亚甲基 [δH 3.37 (d, J = 10.9 Hz);3.34 (d, J = 10.9 Hz), δC 68.8 t];3个甲基 [δH 1.93 (d, J = 0.8 Hz), δC 23.9 q];[δH 1.25 (d, J = 6.4 Hz), δC 23.8 q];(δH 0.96 s, δC 21.9 q)。通过HMBC谱(图 2),比较3个甲基的相关信号,可以推测出连氧的亚甲基可能在C-11或C-12位。由此化合物1的平面结构基本确定。再分析化合物的CD [θ]25 (c 0.5, MeOH):+57 000 (246),−3 600 (319),与已知化合物9-hydroxy- 4,7-megastigmadien-3-one基本一致[14],可以判断6位H的构型为α。在NOESY谱(图 2)中,H-6与连氧亚甲基的2个H分别相关,由此可以确定-OH与C-11相连。综上所述,确定化合物1的结构(图 1),命名为贡山三尖杉倍半萜A(lanceoloside A)。
| 表 1 化合物1的1H-NMR (400 MHz, CD3OD)、13C-NMR (100 MHz, CD3OD) 和DEPT谱数据 Table 1 1H-NMR (400 MHz, CD3OD), 13C-NMR (100 MHz, CD3OD), and DEPT spectroscopic data of compound 1 |
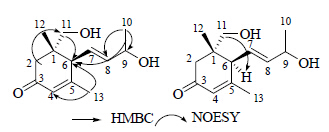 | 图 2 化合物1关键的HMBC 和 NOESY相关信号Fig.2 Key HMBC and NOESY correlations of compound 1 |
化合物2:无色油状物。ESI-MS m/z: 251 [M+Na]+, 227 [M-H]−,推测其相对分子质量为228。结合1H-NMR和13C-NMR推测其分子式为C13H24O3,不饱和度为2。1H-NMR (400 MHz, CD3OD) δ: 1.82 (1H, dd, J = 13.2, 7.1 Hz, H-2a), 1.64 (1H, brt, J = 13.0 Hz, H-2b), 4.17 (1H, m, H-3), 1.98 (1H, dd, J = 13.1, 7.0 Hz, H-4a), 1.56 (1H, dd, J = 13.1, 10.0 Hz, H-4b), 1.35 (1H, m, H-6), 1.49 (1H, m, H-7a), 1.84 (1H, m, H-7b), 1.70 (1H, m, H-8a), 1.78 (1H, m, H-8b), 4.28 (1H, m, H-9), 1.25 (3H, d, J = 6.5 Hz, H-10), 0.94 (3H, s, H-11), 3.46 (1H, dd, J = 8.0, 2.0 Hz, H-12a), 3.76 (1H, d, J = 8.1 Hz, H-12b), 1.16 (3H, s, H-13);13C-NMR (100 MHz, CD3OD) δ: 45.9 (C-1), 49.8 (C-2), 67.1 (C-3), 50.5 (C-4), 86.1 (C-5), 56.8 (C-6), 22.5 (C-7), 40.5 (C-8), 69.1 (C-9), 23.6 (C-10), 20.4 (C-11), 77.3 (C-12), 23.8 (C-13)。以上数据与文献报道基本一致[15],故鉴定化合物2为1个新的天然产物,之前并未命名,故将其命名为贡山三尖杉倍半萜B(lanceoloside B)。
化合物3:无色油状物。ESI-MS m/z: 249 [M+Na]+,推测其相对分子质量为226。结合1H-NMR和13C-NMR推测其分子式为C13H22O3,不饱和度为3。1H-NMR (400 MHz, CD3OD) δ: 1.81 (1H, dd, J = 13.1, 7.0 Hz, H-2a), 1.64 (1H, brt, J = 13.1 Hz, H-2b), 4.17 (1H, m, H-3), 1.97 (1H, dd, J = 13.0, 7.0 Hz, H-4a), 1.55 (1H, dd, J = 13.0, 10.0 Hz, H-4b), 2.04 (1H, d, J = 9.0 Hz, H-6), 5.51 (1H, ddd, J = 15.4, 10.3, 1.1 Hz, H-7), 5.61 (1H, dd, J = 15.4, 5.9 Hz, H-8), 4.28 (1H, m, H-9), 1.24 (3H, d, J = 6.5 Hz, H-10), 0.95 (3H, s, H-11), 3.46 (1H, dd, J = 8.0, 2.0 Hz, H-12a), 3.76 (1H, d, J = 8.0 Hz, H-12b), 1.11 (3H, s, H-13);13C-NMR (100 MHz, CD3OD) δ: 45.5 (C-1), 48.7 (C-2), 67.1 (C-3), 49.3 (C-4), 85.6 (C-5), 61.9 (C-6), 127.5 (C-7), 139.6 (C-8), 69.2 (C-9), 23.9 (C-10), 20.6 (C-11), 77.6 (C-12), 24.1 (C-13)。以上数据与文献报道基本一致[16],故鉴定化合物3为1个新的天然产物,之前未命名,故将其命名为贡山三尖杉倍半萜C(lanceoloside C)。
化合物4:无色油状物。ESI-MS m/z: 231 [M+Na]+, 207 [M-H]−,推测其相对分子质量为208。结合1H-NMR和13C-NMR推测其分子式为C13H20O2,不饱和度为4。1H-NMR (400 MHz, CD3OD) δ: 2.06 (1H, d, J = 16.8 Hz, H-2a), 2.32 (1H, d, J = 16.8 Hz, H-2b), 5.89 (1H, s, H-4), 2.54 (1H, d, J = 8.2 Hz, H-6), 5.54 (1H, dd, J = 15.5, 8.2 Hz, H-7), 5.56 (1H, dd, J = 15.5, 5.6 Hz, H-8), 4.34 (1H, m, H-9), 1.28 (3H, d, J = 6.6 Hz, H-10), 1.03 (3H, s, H-11), 0.97 (3H, s, H-12), 1.88 (3H, d, J = 1.2 Hz, H-13);13C-NMR (100 MHz, CD3OD) δ: 37.3 (C-1), 48.5 (C-2), 202.1 (C-3), 126.3 (C-4), 166.2 (C-5), 56.8 (C-6), 127.3 (C-7), 140.4 (C-8), 68.9 (C-9), 23.9 (C-10), 27.4 (C-11), 28.2 (C-12), 23.8 (C-13)。以上数据与文献报道基本一致[14],故鉴定化合物4为9-hydroxy-4,7-megastigmadien-3-one。
化合物5:无色油状物。ESI-MS m/z: 247 [M+Na]+, 223 [M-H]−,推测相对分子质量为224。结合1H-NMR和13C-NMR推测其分子式为C13H20O3,不饱和度为4。1H-NMR (400 MHz, CD3OD) δ: 2.14 (1H, d, J = 17.2 Hz, H-2a), 2.5 (1H, d, J = 17.0 Hz, H-2b), 5.86 (1H, s, H-4), 5.78 (1H, overlap, H-7), 5.78 (1H, overlap, H-8), 4.31 (1H, m, H-9), 1.22 (3H, d, J = 6.5 Hz, H-10), 1.02 (3H, s, H-11), 0.99 (3H, s, H-12), 1.91 (3H, d, J = 1.4 Hz, H-13);13C-NMR (100 MHz, CD3OD) δ: 42.6 (C-1), 50.8 (C-2), 201.3, (C-3), 127.1 (C-4), 167.6 (C-5), 80.0 (C-6), 130.1 (C-7), 137.0 (C-8), 68.8 (C-9), 24.0 (C-10), 23.9 (C-11), 24.6 (C-12), 19.7 (C-13)。以上数据与文献报道基本一致[17],故鉴定化合物5为corchoionol C。
化合物6:无色油状物。ESI-MS m/z: 247 [M+Na]+, 223 [M-H]−,推测相对分子质量为224。结合1H-NMR和13C-NMR推测其分子式为C13H20O3,不饱和度为4。1H-NMR (400 MHz, CD3OD) δ: 2.09 (1H, d, J = 16.4 Hz, H-2a), 2.33 (1H, d, J = 16.8 Hz, H-2b), 5.91 (1H, s, H-4), 2.55 (1H, d, J = 9.0 Hz, H-6), 5.71 (1H, dd, J = 15.2, 9.0 Hz, H-7), 5.62 (1H, dd, J = 15.2, 5.5 Hz, H-8), 4.30 (1H, m, H-9), 3.69 (1H, dd, J = 11.0, 3.5 Hz, H-10a), 3.50 (1H, dd, J = 11.0, 7.4 Hz, H-10b), 1.03 (3H, s, H-11), 0.96 (3H, s, H-12), 1.90 (3H, d, J = 1.6 Hz, H-13);13C-NMR (100 MHz, CD3OD) δ: 42.3 (C-1), 44.6 (C-2), 201.8 (C-3), 126.8 (C-4), 165.9 (C-5), 50.8 (C-6), 126.5 (C-7), 140.8 (C-8), 68.9 (C-9), 68.8 (C-10), 23.8 (C-11), 23.9 (C-12), 21.9 (C-13)。以上数据与文献报道基本一致[18],故鉴定化合物6为9,10-dihydroxy-4,7-megastigmadien-3-one。
化合物7:无色油状物。ESI-MS m/z: 247 [M+Na]+, 471 [2M+Na]+,推测相对分子质量为224。结合1H-NMR和13C-NMR推测其分子式为C13H20O3,不饱和度为4。1H-NMR (400 MHz, CD3OD) δ: 1.94 (1H, d, J = 16.6 Hz, H-2a), 2.31 (1H, d, J = 16.6 Hz, H-2b), 2.21 (1H, dt, J = 17.1, 1.6 Hz, H-4a), 2.57 (1H, d, J = 17.1 Hz, H-4b), 2.41 (1H, d, J = 9.1 Hz, H-6), 5.74 (1H, overlap, H-7), 5.74 (1H, overlap, H-8), 4.30 (1H, m, H-9), 1.22 (3H, d, J = 6.2 Hz, H-10), 1.03 (3H, s, H-11), 3.61 (1H, dd, J = 8.0, 2.8 Hz, H-12a), 3.68 (1H, d, J = 8.0 Hz, H-12b), 1.16 (3H, s, H-13);13C-NMR (100 MHz, CD3OD) δ: 45.4 (C-1), 49.9 (C-2), 211.8 (C-3), 51.1 (C-4), 85.0 (C-5), 59.6 (C-6), 123.4 (C-7), 143.3 (C-8), 69.0 (C-9), 24.1 (C-10), 20.5 (C-11), 80.0 (C-12), 24.5 (C-13)。以上数据与文献报道基本一致[19],故鉴定化合物7为5,12-epoxy-9- hydroxy-7-megastigmen-3- one。
化合物8:无色油状物。ESI-MS m/z: 263 [M+Na]+, 239 [M-H]−,推测相对分子质量为240。结合1H-NMR和13C-NMR推测其分子式为C13H24O4,不饱和度为4。1H-NMR (400 MHz, CD3OD) δ: 1.91 (1H, d, J = 16.6 Hz, H-2a), 2.35 (1H, d, J = 16.6 Hz, H-2b), 2.21 (1H, dt, J = 17.1, 1.6 Hz, H-4a), 2.57 (1H, d, J = 17.1 Hz, H-4b), 6.08 (1H, d, J = 16.3 Hz, H-7), 6.04 (1H, dd, J = 16.3, 5.1 Hz, H-8), 4.37 (1H, m, H-9), 1.22 (3H, d, J = 6.5 Hz, H-10), 0.96 (3H, s, H-11), 3.67 (1H, dd, J = 8.0, 2.8 Hz, H-12a), 3.75 (1H, d, J = 8.0 Hz, H-12b), 1.12 (3H, s, H-13);13C-NMR (100 MHz, CD3OD) δ: 48.6 (C-1), 52.9 (C-2), 208.7 (C-3), 53.7 (C-4), 82.1 (C-5), 86.5 (C-6), 125.1 (C-7), 140.7 (C-8), 68.2 (C-9), 24.3 (C-10), 15.7 (C-11), 77.9 (C-12), 19.4 (C-13)。以上数据与文献报道基本一致[20],故鉴定化合物8为5,12-epoxy-6,9-hydroxy-7- megastigmen-3-one。
化合物9:无色油状物。ESI-MS m/z: 219 [M+Na]+, 415 [2M+Na]+, 231 [M+Cl]−,推测相对分子质量为196。结合1H-NMR和13C-NMR推测其分子式为C11H16O3,不饱和度为4。1H-NMR (400 MHz, CD3OD) δ: 1.55 (1H, dd, J = 3.6, 14.4 Hz, H-2a), 2.01 (1H, dd, J = 2.4, 14.4 Hz, H-2b), 4.24 (1H, m, H-3), 1.77 (1H, dd, J = 4.0, 13.6 Hz, H-4a), 2.44 (1H, dd, J = 2.8, 13.6 Hz, H-4b), 5.77 (1H, s, H-7), 1.78 (3H, s, H-9), 1.29 (3H, s, H-10), 1.48 (3H, s, H-11);13C-NMR (100 MHz, CD3OD) δ: 37.3 (C-1), 48.0 (C-2), 67.3 (C-3), 46.5 (C-4), 89.0 (C-5), 185.7 (C-6), 113.4 (C-7), 174.5 (C-8), 27.1 (C-9), 27.5 (C-10), 31.1 (C-11)。以上数据与文献报道基本一致[21],故鉴定化合物9为loliolide。
化合物10:无色油状物。ESI-MS m/z: 249 [M+Na]+, 225 [M-H]−,推测相对分子质量为226。结合1H-NMR和13C-NMR推测其分子式为C13H22O3,不饱和度为3。1H-NMR (400 MHz, CD3OD) δ: 1.52 (1H, dd, J = 3.3, 13.8 Hz, H-2a), 2.05 (1H, brd, J = 14.0 Hz, H-2b), 4.49 (1H, m, H-3), 1.98 (1H, dd, J = 4.0, 13.8 Hz, H-4a), 2.62 (1H, brd, J = 14.0 Hz, H-4b), 5.76 (1H, s, H-7), 4.76 (1H, brd, J = 7.2 Hz, H-8), 3.94 (1H, m, H-9), 1.43 (3H, d, J = 6.6 Hz, H-10), 1.42 (3H, s, H-11), 1.13 (3H, s, H-12), 1.92 (3H, s, H-13);13C-NMR (100 MHz, CD3OD) δ: 34.3 (C-1), 51.2 (C-2), 66.5 (C-3), 51.1 (C-4), 89.3 (C-5), 154.6 (C-6), 119.7 (C-7), 88.5 (C-8), 71.3 (C-9), 19.1 (C-10), 27.6 (C-11), 31.3 (C-12), 27.5 (C-13)。以上数据与文献报道基本一致[22],故鉴定化合物10为 (3S,5R,8S)-5,8-epoxy-6-megastigmadien-3,9-diol。
化合物11:无色油状物。ESI-MS m/z: [M+Na]+ 239,推测相对分子质量为238。结合1H-NMR和13C-NMR推测其分子式为C15H26O2,不饱和度为3。1H-NMR (400 MHz, CD3OD) δ: 3.78 (1H, dd, J = 10.6, 5.9 Hz, H-2), 1.60 (1H, m, H-3a), 1.74 (1H, m, H-3b), 1.29 (1H, m, H-5), 1.16 (1H, m, H-6a), 1.06 (1H, m, H-6b), 1.32 (1H, m, H-7a), 1.18 (1H, m, H-7b), 3.24 (1H, brs, H-9), 1.86 (1H, m, H-10a), 1.71 (1H, m, H-10b), 2.47 (1H, m, H-11a), 1.21 (1H, m, H-11b), 1.60 (2H, m, H-12), 1.03 (3H, s, H-13), 0.93 (3H, s, H-14), 1.04 (3H, s, H-15);13C-NMR (100 MHz, CD3OD) δ: 45.6 (C-1), 81.6 (C-2), 48.3 (C-3), 36.0 (C-4), 52.2 (C-5), 27.0 (C-6), 34.6 (C-7), 37.9 (C-8), 76.1 (C-9), 28.0 (C-10), 21.9 (C-11), 36.8 (C-12), 29.3 (C-13), 31.9 (C-14), 25.9 (C-15)。以上数据与文献报道基本一致[23],故鉴定化合物11为clovandiol。
| [1] | 国家中医药管理局《中华本草》编委会. 中华本草 [M]. 上海: 上海科学技术出版社, 1999. |
| [2] | Paudler W W, Kerley G I, McKay J. The Alkaloids of Cephalotaxus drupacea and Cephalotaxus fortunei [J]. J Org Chem, 1963, 28(9): 2194-2197. |
| [3] | Powell R G, Weisleder D, Smith Jr C R, et al. Structures of harringtonine, isoharringtonine, and homoharringtonine [J]. Tetrahedron Lett, 1970, 61(8): 815-818. |
| [4] | Mikolajczak K L, Powell R G, Smith Jr C R. Deoxyharringtonine, a new antitumor alkaloid from Cephalotaxus: Structure and synthetic studies [J]. Tetrahedron, 1972, 28(7): 1995-2001. |
| [5] | Powell R G, Weisleder D, Smith C R. Antitumor alkaloids from Cephalotaxus harringtonia: Structure and activity [J]. J Pharm Sci, 1972, 61(8): 1227-1230. |
| [6] | Paudler W W, McKay J. The structures of some of the minor alkaloids of Cephalotaxus fortunei [J]. J Org Chem, 1973, 38(11): 2110-2112. |
| [7] | 梅文莉, 吴 娇, 戴好富. 三尖杉属植物化学成分与药理活性研究进展 [J]. 中草药, 2006, 37(3): 452-458. |
| [8] | Cardama Q A, Kantarjian H, Cortes J. Homoharringtonine, omacetaxine mepesuccinate, and chronic myeloid leukemia circa [J]. Cancer, 2009, 115: 5382-5393. |
| [9] | Wang L W, Su H J, Yang S Z, et al. New alkaloids and a tetraflavonoid from Cephalotaxus wilsoniana [J]. J Nat Prod, 2004, 67(7): 1182-1185. |
| [10] | He Y R, Shen Y H, Shan L, et al. Diterpenoid lanceolatins A-G from Cephalotaxus lanceolata and their anti- inflammatory and anti-tumor activities [J]. RSC Adv, 2015(5): 4126-4134. |
| [11] | Politi M, Braca A, De Tommasi N, et al. Antimicrobial diterpenes from the seeds of Cephalotaxus harringtonia var. drupacea [J]. Planta Med, 2003, 69(5): 468-469. |
| [12] | Kuo Y H, Lin C H, Hwang S Y, et al. A novel cytotoxic C-methylated biflavone from the stem of Cephalotaxus wilsoniana [J]. Chem Pharm Bull, 2000, 48(3): 440-441. |
| [13] | He Y R, Shen Y H, Li B, et al. Alkaloids from Cephalotaxus lanceolata and their cytotoxicities [J]. Chem Biodivers, 2013, 10(4): 584-595. |
| [14] | D'Abrosca B, DellaGreca M, Fiorentino A, et al. Structure elucidation and phytotoxicity of C13 nor-isoprenoids from Cestrum parqui [J]. Phytochemistry, 2014, 65(4): 497-505. |
| [15] | Matsunami K, Otsuka H, Kondo K, et al. Absolute configuration of (+)-pinoresinol 4-O-[6"-O-galloyl]-β-d- glucopyranoside, macarangiosides E, and F isolated from the leaves of Macaranga tanarius [J]. Phytochemistry, 2009, 70(10): 1277-1285. |
| [16] | Morikawa H, Kasai R, Otsuka H, et al. Terpenic and phenolic glycosides from leaves of Breynia officinalis Hemsl [J]. Chem Pharm Bull, 2004, 52(9): 1086-1090. |
| [17] | Gonzalez A G, Guillermo J A, Ravelo A G, et al. 4, 5-Dihydroblumenol A, a new nor-isoprenoid from Perrottetia multiflora [J]. J Nat Prod, 1994, 57(3): 400-402. |
| [18] | Greger H, Pacher T, Brem B, et al. Insecticidal flavaglines and other compounds from Fijian Aglaia species [J]. Phytochemistry, 2001, 57(1): 57-64. |
| [19] | Fernández I, Pedro J R, Vidal R. Norisoprenoids from Centaurea aspera and C. salmantica. [J]. Phytochemistry, 1993, 34(3): 733-736. |
| [20] | Powell R G, Smith Jr C R. An investigation of the antitumor activity of Sesbania drummondii [J]. J Nat Prod, 1981, 44(1): 86-90. |
| [21] | Valdes III L J. Loliolide from Salvia divinorum [J]. J Nat Prod, 1986, 49(1): 171. |
| [22] | Marukami T, Kishi A, Yoshikawa M. Medicinal flowers. IV. Marigold. (2): Structures of new ionone and sesquiterpene glycosides from Egyptian Calendula officinalis [J]. Chem Pharm Bull, 2001, 49(8): 974-978. |
| [23] | Shi J G, Shi Y P, Jia Z J. Sesquiterpenoids from Euphorbia wangii [J]. Phytochemistry, 1997, 45(2): 343-347. |
 2015, Vol. 46
2015, Vol. 46


