2. 中山大学药学院, 广东 广州 510080
2. School of Pharmaceutical Sciences, Sun Yat-sen University, Guangzhou 510080, China
岩黄连又名岩连,系罂粟科(Papaveraceae)紫堇属Corydalis DC.多年生草本植物岩黄连Corydalis saxicola Bunting的全草,被收载于《广西中药材标准》[1],据《广西植物志》、《贵州本草》、《中药大辞典》、《新华本草纲要》等所记载:本品性苦、寒,具有清热、消肿、止血、止痛、利湿、拔毒等作用,民间用于口舌糜烂、目赤、急性腹痛、肝炎、肝硬化、痢疾、痔疮出血、晚期癌痛、急性黄疸型肝炎等症[2]。近代药理学研究表明岩黄连能够用于流行性热毒病、瘟疫、毒痢、痛肿疮、急慢性肝炎、乙型肝炎、丙型肝炎、丁型肝炎、肝硬化、肝脓疡、肝腹水、胆囊炎、肝癌等症[3]。根据文献报道[4, 5, 6],小檗碱能提高脂蛋白酶的活性,促进LDL受体的表达,通过腺苷酸酶(AMP)活性抑制脂质的合成以及减少过氧化物酶体增殖活化受体(PPARγ)的表达,从而表现出一定的抗氧化活性。
本课题前期研究表明岩黄连70%乙醇提取物以及各萃取层均显示出良好的抗氧化活性,但国内外未见相关的岩黄连抗氧化活性实验报道,因此,本研究对岩黄连中单体成分进行系统的分离纯化研究,从岩黄连全草70%乙醇提取物中得到16个化合物,分别鉴定为卡维汀(cavidine,1)、刺罂粟碱(stylopine,2)、氢化小檗碱(canadine,3)、四氢巴马汀(tetrahydropalmatine,4)、碎叶紫堇碱(cheilanthifoline,5)、斯氏紫堇碱(scoulerine,6)、原阿片碱(protopine,7)、去氢碎叶紫堇碱(dehydro- cheilanthifoline,8)、脱氢异阿朴卡维汀(dehydro- isoapocavidine,9)、小檗碱(berberine,10)、去氢分离木瓣树胺(dehydrodiscretamine,11)、白屈菜红碱(chelerythrine,12)、脱氢卡维汀(dehydrocavidine,13)、黄堇碱(corypalline,14)、异紫堇定碱(isocorydine,15)、深山黄堇碱(pallidine,16)。其中化合物3、5、8、11、16为首次从该植物中分离得到。采用DPPH清除法进行抗氧化活性筛查,为岩黄连作为天然抗氧化剂的开发提供实验依据。
1 仪器与材料X—4熔点仪(北京泰克仪器有限公司),Brucker EQUINOX55傅里叶变换红外光谱-红外显微镜联用仪(Brucker公司),Bruker Avance AV 400超导核磁共振谱仪(Brucker公司),Brucker microOTOF-Q质谱仪(Brucker公司),WZZ—2S自动旋光仪(上海精密科学仪器有限公司)。硅胶H(200~300目,Merck);大孔树脂Diaion HP-20(Mitsubishi Chemical Ltd.,日本);MCI gel CHP-20P(75~150 μm,Mitsubishi Chemical Ltd.,日本);反相硅胶Chromatorex RP18(100~200目,Fuji Silysia Chemical Ltd.,日本);阳离子交换树脂Amberlite IR-120(plus)(Sigma公司);TLC硅胶预制板:Silica gel GF254(Merck公司);所用其他试剂均为分析纯。
岩黄连全草于2009年5月采自广西壮族自治区东兰县GAP种植基地,人工栽培品种,阴干后备用。经中山大学药学院生药学实验室杨得坡教授鉴定为罂粟科紫堇属植物岩黄连Corydalis saxicola Bunting,标本保存于中山大学药学院生药学实验室。
2 方法 2.1 提取与分离岩黄连的干燥全草3 kg,粉碎(过40目筛),用8倍量的70%乙醇超声提取3次,每次提取30 min,减压回收溶剂后,将所得的流浸膏均匀分散于适量的水溶液中,分别用等体积的石油醚、醋酸乙酯、水饱和的正丁醇萃取,得到石油醚萃取部分(8.34 g)、醋酸乙酯萃取部分(10.21 g)、正丁醇萃取部分(80.94 g)和水层留余部分(200.69 g)。
石油醚萃取部分经硅胶柱色谱(环己烷-醋酸乙酯100∶0→92∶8),得到化合物1(41 mg)和2(32 mg)。醋酸乙酯萃取部分经硅胶柱色谱(氯仿-甲醇100∶0→95∶5),得到3个馏份,再分别经(环己烷-醋酸乙酯)得到化合物3(15 mg)、4(17 mg)、5(8 mg)。正丁醇萃取部分经ODS反相硅胶柱色谱(甲醇-水)以及硅胶柱色谱(氯仿-甲醇)预处理后,再经硅胶柱色谱(正己烷-醋酸乙酯-乙醇)以及重结晶手段,得到化合物6(26 mg)、7(11 mg)、8(21 mg)、9(13 mg)、10(18 mg)、11(14 mg)和12(5 mg)。水层留余部分置于0~4 ℃冰箱内放置,析出黄色粉末,滤出粉末经过制备高效液相色谱纯化,得到化合物13(71 mg)。水溶液经Diaion HP-20(甲醇-水)、以及反复硅胶柱色谱后,得到化合物14(7 mg)、15(11 mg)、16(7 mg)。
2.2 抗氧化活性测定精密称取DPPH 3.94 mg置于50 mL棕色量瓶,用95%乙醇溶解并稀释至刻度,配制浓度为200 μmol/mL的DPPH乙醇溶液,避光保存。精密量取样品试液1 mL、95%乙醇2 mL、200 μmol/mL DPPH试液2 mL作为实验组,另取样品试液1 mL、95%乙醇4 mL作为空白组,95%乙醇3 mL、DPPH试液2.0 mL作为对照组。各组溶液分别于试管内混匀,室温条件下避光反应30 min,在517 nm处测定其吸光度(A)值。按照下式计算各待测物的自由基清除率。
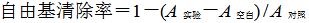
数据采用SPSS 15.0软件进行统计分析,IC50采用Origin 7.5软件进行计算。
3 结果与讨论 3.1 结构鉴定化合物1:黄色无定形粉末,mp 189~190 ℃,C21H23NO4,ESI-MS m/z: 354 [M+H]+。1H-NMR (300 MHz,CDCl3) δ: 0.94 (3H,d,J = 6.9 Hz,13-CH3),2.55 (1H,dd,J = 3.6,15.0 Hz,H-6α),2.61 (1H,d,J = 15.0 Hz,H-5α),3.10 (1H,m,H-5β),3.15 (1H,m,H-6β),3.24 (1H,q,J = 3.0,6.6 Hz,H-13),3.50 (1H,d,J = 15.0 Hz,H-8α),3.51 (1H,d,J = 15.0 Hz,H-14),3.86 (3H,s,2-OCH3),3.88 (3H,s,3-OCH3),4.07 (1H,dd,J = 6.0,15.0 Hz,H-8β),5.92 (2H,dd,J = 1.5,11.0 Hz,-OCH2O-),6.57 (1H,s,H-4),6.64 (1H,d,J = 8.0 Hz,H-11),6.67 (1H,s,H-1),6.70 (1H,d,J = 8.0 Hz,H-12);13C-NMR (75 MHz,CDCl3) δ: 17.8 (13-CH3),28.9 (C-5),38.2 (C-13),51.0 (C-6),52.9 (C-8),55.2 (2-OCH3),55.7 (3-OCH3),62.6 (C-14),100.8 (-OCH2O-),106.3 (C-11),108.1 (C-1),109.2 (C-4),110.5 (C-8a),116.4 (C-12),120.7 (C-4a),127.8 (C-14a),135.3 (C-12a),143.2 (C-10),144.0 (C-9),146.9 (C-2),147.5 (C-3)。上述数据与文献报道一致[7],故鉴定化合物1为卡维汀。
化合物2:黄色无定形粉末,mp 194~195 ℃,C19H17NO4,ESI-MS m/z: 324 [M+H]+。1H-NMR (300 MHz,CDCl3) δ: 2.67 (1H,dd,J = 3.0,10.0 Hz,H-6α),2.71 (1H,d,J = 3.0 Hz,H-5α),2.88 (1H,dd,J = 4.5,11.4 Hz,H-13α),3.18 (1H,m,H-5β),3.22 (1H,m,H-6β),3.27 (1H,dd,J = 4.0,15.9 Hz,H-13β),3.60 (1H,d,J = 15.0 Hz,H-8),3.64 (1H,d,J = 15.0 Hz,H-14),4.13 (1H,d,J = 15.0 Hz,H-8a),5.95 (2H,s,9,10-OCH2O-),6.00 (2H,s,2,3-OCH2O-),6.62 (1H,s,H-4),6.67 (1H,d,J = 8.0 Hz,H-11),6.72 (1H,d,J = 8.0 Hz,H-12),6.76 (1H,s,H-1);13C-NMR (75 MHz,CDCl3) δ: 30.9 (C-5),37.6 (C-13),52.3 (C-6),54.0 (C-8),60.9 (C-14),101.7 (2,3-OCH2O-),102.3 (9,10-OCH2O-),106.5 (C-1),107.8 (C-11),109.4 (C-4),117.7 (C-8a),121.9 (C-12),128.7 (C-4a),129.5 (12a),131.7 (14a),144.4 (C-10),146.0 (C-2),147.2 (C-3),147.5 (C-9)。上述数据与文献报道一致[8],故鉴定化合物2为刺罂粟碱。
化合物3:黄色无定形粉末,mp 165~166 ℃,C20H21NO4,ESI-MS m/z: 340 [M+H]+。1H-NMR (300 MHz,CDCl3) δ: 2.64 (1H,m,H-5α),2.66 (1H,d,J = 12.6 Hz,H-6α),2.83 (1H,dd,J = 11.4,15.6 Hz,H-13α),3.12 (1H,m,H-5β),3.18 (1H,m,H-13β),3.23 (1H,dd,J = 3.6,15.9 Hz,H-6β),3.55 (1H,d,J = 15.1 Hz,H-8α),3.55 (1H,d,J = 15.1 Hz,H-14),3.85 (6H,s,9,10-OCH3),4.24 (1H,d,J = 15.9 Hz,H-8β),5.91 (2H,s,-OCH2O-),6.58 (1H,s,H-4),6.72 (1H,s,H-1),6.78 (1H,d,J = 8.0 Hz,H-11),6.85 (1H,d,J = 8.0 Hz,H-12);13C-NMR (75 MHz,CDCl3) δ: 29.8 (C-5),36.7 (C-13),51.7 (C-6),54.2 (C-8),56.2 (10-OCH3),59.9 (9-OCH3),60.5 (C-14),101.0 (2,3-OCH2O-),105.7 (C-1),108.6 (C-4),111.3 (C-11),124.1 (C-12),127.8 (C-12a),127.9 (C-4a),128.6 (C-8a),130.9 (C-14a),145.3 (C-9),146.1 (C-3),146.3 (C-2),150.5 (C-10)。上述数据与文献报道一致[9],故鉴定化合物3为氢化小檗碱。
化合物4:黄色无定形粉末,mp 139~140 ℃,C21H25NO4,ESI-MS m/z: 356 [M+H]+。1H-NMR (300 MHz,CDCl3) δ: 2.63 (1H,m,H-6α),2.67 (1H,m,H-5α),2.84 (1H,dd,J = 4.2,11.4 Hz,H-13α),3.11 (1H,dd,J = 6.0,16.5 Hz,H-5β),3.18 (1H,dd,J = 5.4,9.6 Hz,H-6β),3.27 (1H,dd,J = 3.6,15.9 Hz,H-13β),3.54 (1H,d,J = 15.3 Hz,H-8α),3.54 (1H,d,J = 15.3 Hz,H-14),3.84 (3H,s,9-OCH3),3.85 (3H,s,2-OCH3),3.86 (3H,s,10-OCH3),3.88 (3H,s,3-OCH3),4.24 (1H,d,J = 15.9 Hz,H-8β),6.61 (1H,s,H-4),6.72 (1H,s,H-1),6.78 (1H,d,J = 8.0 Hz,H-11),6.87 (1H,d,J = 8.0 Hz,H-12);13C-NMR (75 MHz,CDCl3)δ: 29.4 (C-5),36.6 (C-13),51.8 (C-6),54.3 (C-8),56.2 (2-OCH3),56.2 (9-OCH3),56.4 (3-OCH3),59.6 (10-OCH3),60.5 (C-14),108.9 (C-1),111.3 (C-11),111.7 (C-4),124.0 (C-12),127.0 (C-4a),128.0 (C-8a),128.8 (C-12a),129.9 (C-14a),145.3 (C-9),147.6 (C-2),147.7 (C-3),150.4 (C-10)。上述数据与文献报道基本一致[9],故鉴定化合物4为四氢巴马汀。
化合物5:黄色无定形粉末,mp 176~178 ℃,C19H19NO4,ESI-MS m/z: 326 [M+H]+。1H-NMR (300 MHz,CDCl3) δ: 2.65 (1H,d,J = 11.7 Hz,H-6α),2.69 (1H,d,J = 11.7 Hz,H-5α),2.81 (1H,dd,J = 4.2,11.7 Hz,H-13α),3.15 (1H,d,J = 9.9 Hz,H-5β),3.19 (1H,d,J = 9.9 Hz,H-6β),3.26 (1H,dd,J = 3.3,15.9 Hz,H-13β),3.54 (1H,d,J = 15.3 Hz,H-8α),3.54 (1H,m,H-14),3.87 (3H,s,3-OCH3),4.10 (1H,d,J = 15.3 Hz,H-8β),5.94 (2H,dd,J = 10.2,10.2 Hz,-OCH2O-),6.59 (1H,s,H-4),6.63 (1H,d,J = 8.0 Hz,H-11),6.68 (1H,d,J = 8.0 Hz,H-12),6.81 (1H,s,H-1);13C-NMR (75 MHz,CDCl3) δ: 29.5 (C-5),36.6 (C-13),51.8 (C-6),53.3 (C-8),56.2 (3-OCH3),59.7 (C-14),101.2 (9,10-OCH2O-),107.0 (C-1),110.9 (C-4),111.6 (C-11),117.0 (C-8a),121.3 (C-12),126.1 (C-14a),128.8 (C-12a),130.5 (C-4a),143.4 (C-10),144.1 (C-9),145.1 (C-3),145.3 (C-2)。上述数据与文献报道一致[10],故鉴定化合物5为碎叶紫堇碱。
化合物6:无定形粉末,C19H21NO4,ESI-MS m/z: 328 [M+H]+。1H-NMR (300 MHz,CDCl3) δ: 2.63 (1H,m,H-6α),2.67 (1H,d,J = 12.3 Hz,H-5α),2.83 (1H,dd,J = 11.7,15.0 Hz,H-13α),3.11 (1H,m,H-5β),3.18 (1H,m,H-6β),3.21 (1H,dd,J = 4.2,15.0 Hz,H-13β),3.53 (2H,d,J = 15.0 Hz,H-8α,14),3.86 (6H,s,3,10-OCH3),4.24 (1H,d,J = 15.0 Hz,H-8β),6.59 (1H,s,H-4),6.66 (1H,d,J = 8.1 Hz,H-12),6.72 (1H,d,J = 8.1 Hz,H-11),6.81 (1H,s,H-1);13C-NMR (75 MHz,CDCl3) δ: 29.5 (C-5),36.6 (C-13),51.9 (C-6),53.8 (C-8),56.2 (10-OCH3),56.5 (3-OCH3),59.5 (C-14),109.2 (C-1),110.9 (C-11),111.7 (C-4),119.5 (C-12),121.4 (C-8a),126.3 (C-14a),128.4 (C-12a),130.8 (C-4a),141.7 (C-9),144.2 (C-3),144.4 (C-10),145.3 (C-2)。上述数据与文献报道一致[10],故鉴定化合物6为斯氏紫堇碱。
化合物7:白色无定形粉末,C20H19NO5,ESI-MS m/z: 354 [M+H]+。1H-NMR (300 MHz,CDCl3) δ: 1.94 (3H,s,N-CH3),2.55 (2H,brs,H-5),3.59 (2H,br s,H-8),3.79 (2H,brs,H-13),5.92 (2H,s,9,10-OCH2O-),5.95 (2H,s,2,3-OCH2O-),6.64 (1H,s,H-4),6.66 (1H,d,J = 8.0 Hz,H-12),6.67 (1H,d,J = 8.0 Hz,H-11),6.90 (1H,s,H-1);13C-NMR (75 MHz,CDCl3) δ: 32.1 (C-5),41.8 (N-CH3),46.8 (C-13),51.1 (C-8),58.1 (C-6),101.1 (9,10-OCH2O-),101.4 (2,3-OCH2O-),107.0 (C-11),108.4 (C-1),110.7 (C-4),118.1 (C-8a),125.3 (C-12),129.1 (C-12a),132.9 (C-4a),136.3 (C-14a),146.1 (C-9),146.2 (C-10),146.5 (C-2),148.2 (C-3),180.1 (C-14)。上述数据与文献报道基本一致[11],故鉴定化合物7为原阿片碱。
化合物8:橘色针晶,C19H16NO4,ESI-MS m/z: 322 [M]+。1H-NMR (300 MHz,DMSO-d6) δ: 3.18 (2H,t,J = 5.1 Hz,H-5),3.88 (3H,s,3-OCH3),4.87 (2H,t,J = 5.1 Hz,H-6),6.50 (2H,s,-OCH2O-),7.02 (1H,s,H-4),7.49 (1H,s,H-1),7.84 (1H,d,J = 8.7 Hz,H-11),7.98 (1H,d,J = 8.7 Hz,H-12),8.79 (1H,s,H-13),9.35 (1H,s,H-8),9.89 (1H,s,2-OH);13C-NMR (75 MHz,DMSO-d6) δ: 26.8 (C-5),56.2 (C-6),56.7 (3-OCH3),105.1 (9,10-OCH2O-),112.1 (C-12),112.3 (C-1),112.8 (C-11),119.8 (C-14a),121.1 (C-12),121.5 (C-13),122.4 (C-14),127.6 (C-12a),133.1 (C-8a),137.8 (C-4a),144.3 (C-8),145.1 (C-10),147.0 (C-9),147.5 (C-3),151.2 (C-2)。上述数据与文献报道一致[12],故鉴定化合物8为去氢碎叶紫堇碱。
化合物9:黄色无定形粉末,C20H18NO4。ESI-MS m/z: 336 [M]+。1H-NMR (300 MHz,DMSO-d6)δ: 2.88 (3H,s,13-CH3),3.18 (2H,t,J = 5.4 Hz,H-5),3.88 (3H,s,2-OCH3),4.86 (2H,t,J = 5.4 Hz,H-6),6.52 (2H,s,-OCH2O-),7.06 (1H,s,H-4),7.75 (1H,s,H-1),7.80 (1H,d,J = 8.7 Hz,H-12),8.02 (1H,d,J = 8.7 Hz,H-11),9.92 (1H,s,H-8);13C-NMR (75 MHz,DMSO-d6) δ: 18.2 (13-CH3),26.3 (C-5),55.0 (C-6),55.8 (2-OCH3),104.3 (-OCH2O-),105.1 (C-1),108.3 (C-4),111.5 (C-8a),119.0 (C-14a),120.8 (C-11),121.5 (C-12),130.4 (C-4a),131.7 (C-12),132.1 (C-13),136.6 (C-14),143.6 (C-8),144.3 (C-9),146.8 (C-10),147.5 (C-3),149.5 (C-2)。上述数据与文献报道一致[13],故鉴定化合物9为脱氢异阿朴卡维汀。
化合物10:黄色无定形粉末,C20H18NO4。ESI-MS m/z: 336 [M]+。1H-NMR (300 MHz,D2O) δ: 2.87 (2H,brs,H-5),3.78 (3H,s,10-OCH3),3.89 (3H,s,9-OCH3),4.53 (2H,m,H-6),5.76 (2H,s,2,3-OCH2O-),6.55 (1H,s,H-4),6.72 (1H,s,H-1),7.34 (1H,d,J = 9.0 Hz,H-12),7.58 (1H,d,J = 9.0 Hz,H-11),7.67 (1H,s,H-13),9.28 (1H,s,H-8);13C-NMR (75 MHz,D2O) δ: 26.4 (C-5),56.0 (C-6),56.8 (10-OCH3),62.4 (9-OCH3),102.5 (-OCH2O-),104.5 (C-1),108.5 (C-4),119.3 (C-13),119.6 (C-14a),121.4 (C-8a),123.5 (C-12),126.4 (C-11),128.1 (C-4a),132.9 (C-12a),137.2 (C-14),143.2 (C-9),144.1 (C-8),147.0 (C-2),150.5 (C-3),151.0 (C-10)。上述数据与文献报道一致[14],故鉴定化合物10为小檗碱。
化合物11:红色无定形粉末,C19H18NO4。ESI-MS m/z: 324 [M]+。1H-NMR (300 MHz,CF3COOD)δ: 3.31 (2H,t,J = 5.0 Hz,H-5),4.03 (3H,s,3-OCH3),4.13 (3H,s,10-OCH3),4.88 (2H,t,J = 6.0 Hz,H-6),6.97 (1H,s,H-4),7.64 (1H,s,H-1),7.73 (1H,d,J = 9.0 Hz,H-11),7.92 (1H,d,J = 9.0 Hz,H-12),8.36 (1H,s,H-13),9.54 (1H,s,H-8);13C-NMR (75 MHz,CF3COOD) δ: 29.3 (C-5),57.9 (3-OCH3),58.7 (10-OCH3),58.9 (C-6),112.9 (C-1),113.7 (C-4),121.8 (C-11),121.9 (C-14a),122.6 (C-12),126.9 (C-13),131.0 (C-12a),134.3 (C-14),135.4 (C-8a),140.1 (C-4a),145.0 (C-10),146.0 (C-8),146.8 (C-9),147.7 (C-3),152.7 (C-2)。上述数据与文献报道一致[15],故鉴定化合物11为去氢分离木瓣树胺。
化合物12:浅黄色无定形粉末,C21H18NO4。ESI-MS m/z: 348 [M]+。1H-NMR (300 MHz,DMSO-d6) δ: 2.49 (3H,s,N-CH3),3.82 (3H,s,7-OCH3),3.87 (3H,s,8-OCH3),5.42 (1H,s,6-H),6.13 (2H,s,-OCH2O-),7.17 (1H,d,J = 8.7 Hz,H-9),7.30 (1H,s,H-1),7.52 (1H,d,J = 8.7 Hz,H-12),7.57 (1H,s,H-4),7.70 (1H,d,J = 8.7 Hz,H-10),7.83 (1H,d,J = 8.7 Hz,H-11);13C-NMR (75 MHz,DMSO-d6) δ: 39.4 (N-CH3),56.6 (8-OCH3),61.6 (7-OCH3),84.6 (C-6),100.5 (C-4),101.9 (-OCH2O-),105.1 (C-1),113.9 (C-9),119.4 (C-10),120.4 (C-11),122.8 (C-10b),123.9 (C-12),124.8 (C-10a),126.0 (C-4a),126.7 (C-6a),131.2 (C-12a),138.8 (C-4b),146.6 (C-7),147.8 (C-2),148.4 (C-3),152.4 (C-8)。上述数据与文献报道一致[16],故鉴定化合物12为白屈菜红碱。
化合物13:黄色无定形粉末,C21H20NO4。ESI-MS m/z: 350 [M]+。1H-NMR (300 MHz,DMSO-d6) δ: 2.96 (1H,s,13-CH3),3.13 (2H,t,J = 9.0 Hz,H-5),3.84 (3H,s,-OCH3),3.88 (3H,s,-OCH3),4.78 (2H,t,J = 9.0 Hz,H-6),6.54 (2H,s,-OCH2O-),7.15 (1H,s,H-1),7.36 (1H,s,H-4),7.98 (1H,d,J = 9.0 Hz,H-12),8.04 (1H,d,J = 9.0 Hz,H-11),9.92 (1H,s,H-8);13C-NMR (75 MHz,DMSO-d6) δ: 19.0 (13-CH3),27.6 (C-5),56.6 (-OCH3),57.0 (-OCH3),57.5 (C-6),105.4 (-OCH2O-),111.6 (C-4),111.7 (C-1),115.2 (C-11),119.8 (C-12),120.1 (C-13),120.8 (C-14a),131.1 (C-14),132.4 (C-12a),133.1 (C-8a),136.3 (C-4a),143.7 (C-8),145.3 (C-10),147.6 (C-9),147.8 (C-3),151.3 (C-2)。上述数据与文献报道一致[17],故鉴定化合物13为脱氢卡维汀。
化合物14:黄色无定形粉末,C11H14NO2。ESI-MS m/z: 193 [M+H]+。1H-NMR (400 MHz,DMSO-d6) δ: 2.30 (3H,s,N-CH3),2.53 (2H,t,J = 5.6 Hz,H-4),2.68 (2H,t,J = 5.6 Hz,H-3),3.47 (2H,brs,H-1),3.71 (3H,s,6-OCH3),6.44 (1H,s,H-8),6.61 (1H,s,H-5);13C-NMR (100 MHz,DMSO-d6): 28.7 (C-4),46.1 (N-CH3),53.1 (C-3),56.1 (6-OCH3),57.3 (C-1),112.7 (C-5),113.6 (C-8),124.5 (C-4a),127.4 (C-8a),144.9 (C-7),146.7 (C-6)。上述数据与文献报道一致[18],故鉴定化合物14为黄堇碱。
化合物15:淡黄色无定形粉末,C20H23NO4。ESI-MS m/z: 342 [M+H]+。1H-NMR (300 MHz,DMSO-d6) δ: 2.57 (2H,d,J = 13.2 Hz,H-7),2.76 (2H,d,J = 16.5 Hz,H-4),2.86 (3H,s,N-CH3),3.08 (2H,d,J = 11.7 Hz,H-5),3.37 (3H,s,1-OCH3),3.65 (3H,s,10-OCH3),3.67 (3H,s,2-OCH3),4.28 (1H,d,J = 13.2 Hz,H-6a),6.33 (1H,d,J = 7.8 Hz,H-8),6.46 (1H,s,H-3),6.58 (1H,d,J = 7.5 Hz,H-9);13C-NMR (75 MHz,DMSO-d6) δ: 24.0 (C-4),31.3 (C-7),43.3 (N-CH3),53.4 (C-5),56.0 (10-OCH3),56.5 (2-OCH3),61.2 (1-OCH3),69.9 (C-6a),109.4 (C-3),110.5 (C-9),112.4 (C-8),113.2 (C-11a),120.7 (C-11b),123.3 (C-3a),123.7 (C-6b),125.8 (C-7a),150.9 (C-1),151.8 (C-11),152.6 (C-10),153.0 (C-2)。上述数据与文献报道一致[19],故鉴定化合物15为异紫堇定碱。
化合物16:淡黄色无定形粉末,C19H21NO4。HR-ESI-MS m/z: 328.160 4 [M+H]+ (计算值 328.154 33)。1H-NMR (400 MHz,CDCl3) δ: 1.83 (1H,m,H-15),1.94 (1H,m,H-15),2.45 (3H,s,N-CH3),2.59 (2H,m,H-16),3.00 (1H,dd,J = 4.8,13.5 Hz,H-10),3.31 (1H,d,J = 13.5 Hz,H-10),3.68 (1H,dd,J = 6.8,12.8 Hz,H-9),3.80 (3H,s,6-OCH3),3.89 (3H,s,3-OCH3),6.31 (1H,s,H-8),6.34 (1H,s,H-5),6.70 (1H,s,H-1),6.78 (1H,s,H-4);13C-NMR (100 MHz,CDCl3) δ: 32.4 (C-10),41.3 (C-15),41.6 (N-CH3),42.3 (C-13),45.7 (C-16),55.1 (6-OCH3),56.2 (3-OCH3),60.8 (C-9),107.6 (C-4),113.6 (C-1),118.9 (C-5),122.3 (C-8),129.3 (C-11),129.6 (C-12),144.9 (C-2),145.8 (C-3),151.4 (C-6),161.6 (C-14),180.9 (C-7)。上述数据与文献报道一致[20],故鉴定化合物16为深山黄堇碱。
3.2 抗氧化活性岩黄连中9种生物碱的体外清除自由基活性结果见表 1,其IC50在0.25~16.51 mg/mL。
|
|
表 1 岩黄连中9种生物碱体外清除自由基活性检测结果 Table 1 Determination of nine alkaloids from C. saxicola on in vitro scavenging free radical of DPPH |
进行抗氧化活性构效关系研究,分别比较考察3对结构类似的叔胺碱与季胺碱(碎叶紫堇碱和去氢碎叶紫堇碱、卡维汀和脱氢卡维汀、氢化小檗碱与小檗碱)清除DPPH自由基活性的能力,结果表明叔胺碱的抗氧化活性要高于其相应的季胺碱,其原因可能是叔胺碱上的N原子可提供一对电子,供自由基结合,从而提高其抗氧化活性。
比较考察碎叶紫堇碱与氢化小檗碱二者清除DPPH自由基活性的能力,结果表明碎叶紫堇碱的活性高于氢化小檗碱,原因可能是碎叶紫堇碱的2位为酚羟基,而氢化小檗碱的2位为甲氧基,酚羟基可电离成氧负离子,氧负离子可提供一对电子与自由基进行结合,可提高其抗氧化活性。
比较考察氢化小檗碱与卡维汀清除DPPH自由基活性的能力,结果表明氢化小檗碱的活性高于卡维汀,原因可能是13位上甲基的存在增加了化合物与自由基结合的位阻,从而导致抗氧化活性的下降。
考察岩黄连中9种生物碱清除DPPH自由基的动力学模型,活性最高的2个化合物(碎叶紫堇碱与深山黄堇碱)为非线性清除DPPH自由基,属于S型曲线模型,具体原因尚待考察,其他生物碱清除DPPH自由基的模型为线性,表明其DPPH自由基清除能力呈剂量依赖性。
因此,岩黄连具有良好的抗氧化活性物质基础,研究结果可为岩黄连作为一种抗炎中药的开发提供一定的参考价值。
| [1] | 广西中药材标准[S]. 1990. |
| [2] | 韦记青, 蒋水元, 蒋运生, 等, 药用植物岩黄连研究概述[J]. 广西科学院学报, 2006, 22(2): 108-111. |
| [3] | 孙宁玲, 陆国才, 袁伯俊, 等. 岩黄连研究进展[J]. 中药新药与临床药理, 2006, 17(1): 78-80. |
| [4] | Kong W, Wei J, Abidi P, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins[J]. Nat Med, 2004, 10(12): 1344-1351. |
| [5] | Brusq J M, Ancellin N, Grondin P, et al. Inhibition of lipid synthesis through activation of AMP kinase: an additional mechanism for the hypolipidemic effects of berberine[J]. J Lipid Res, 2006, 47(6): 1281-1288. |
| [6] | Huang C, Zhang Y B, Gong Z W, et al. Berberine inhibits 3T3-L1 adipocyte differentiation through the PPAR gamma pathway[J]. Biochem Biophys Res Commun, 2006, 348(2): 571-578. |
| [7] | 柯珉珉, 张宪德, 吴练中, 等. 岩黄连有效成分的研究[J]. 植物学报, 1982, 24(3): 289-291. |
| [8] | Chrzanowska M. Synthesis of isoquinoline alkaloids, total synthesis of (±)-stylopine[J]. J Nat Prod, 1995, 58: 401-407. |
| [9] | Saito S, Tanaka M, Matsunaga K, et al. The combination of rat mast cell and rabbit aortic smooth muscle is the simple bioassay for the screening of anti-allergic ingreditent from methanolic extract of corydalis tuber[J]. Biol Pharm Bull, 2004, 27(8): 1270-1274. |
| [10] | Wangchuk P, Keller P A, Pyne S G, et al. Antimalarial alkaloids from a Bhutanese traditional medicinal plant Corydalis dubia[J]. J Ethnopharmacol, 2012, 143(1): 310-313. |
| [11] | Seger C, Sturm S, Strasser E M, et al. 1H and 13C-NMR signal assignment of benzylisoquinoline alkaloids from Fumaria officinalis L. (Papaveraceae).[J]. Magn Reson. Chem, 2004, 42(10): 882-886. |
| [12] | Pandey V B, Ray A B, Dasgupta B. Quaternary alkaloids of Fumaria indica[J]. Phytochemistry, 1976, 15: 545-546. |
| [13] | 李慧梁. 岩黄连活性成分系统研究及藜芦毒性成分研究.[D]. 上海: 第二军医大学, 2006. |
| [14] | Huang M J, Lee K S, Hurley S J. Nuclear magnetic resonance spectral analysis and molecular properties of berberine[J]. Int J Quantum Chem, 2005, 105(4): 396-409. |
| [15] | Chattopadhyay S K, Ray A B, Slatkin D J, et al. Quaternary alkaloids of Thalictrum foliolosum[J]. Phytochemistry, 1983, 22(11): 2607-2610. |
| [16] | Seckarova P, Marek R, Dostal J, et al. Structural studies of benzophenanthridine alkaloid free bases by NMR spectroscopy[J]. Magn Reson Chem, 2002, 40: 147-152. |
| [17] | Bhakuni D S, Chaturvedi R. The alkaloids of Corydalis meifolia[J]. J Nat Prod, 1983, 46(3): 321-324. |
| [18] | Jendrzejewski S. Degradation of reticuline in Berberis suspension cultures[J]. Phytochemistry, 1990, 29(1): 135-139. |
| [19] | 徐 蔚, 宋启示, 王 培, 等. 对叶榕叶和细枝的化学成分研究[J]. 天然产物研究与开发, 2010, 22(6): 1003-1005. |
| [20] | Gan L S, Yao W, Mo J X, et al. Alkaloids from Lindera aggregate[J]. Nat Prod Commun, 2009, 4(1): 43-49. |
 2014, Vol. 45
2014, Vol. 45


