2. 基于靶点的药物设计与研究教育部重点实验室, 辽宁 沈阳 110016;
3. 大连大学生命科学与技术学院, 辽宁 大连 116622
2. Key Laboratory of Structure-Based Drug Design & Discovery of Ministry of Education, Shenyang Pharmaceutical University, Shenyang 110016, China;
3. School of Life Sciences and Biotechnology, Dalian University, Dalian 116622, China
姜黄Curcummae Longae Rhizoma是姜科(Zingiberaceae)姜黄属Zingiber Boehmer植物姜黄Curcuma longa L. 的干燥根茎,为多年生草本植物。姜黄辛、苦、温,归脾、肝经,有破血行气、通经止痛的功效。姜黄既可当作药用,亦可当作着色剂、调味品及防腐剂。研究发现姜黄的药用部位含有多种结构类型的化学成分,倍半萜和二苯基庚酮类为主要的化学成分[1]。研究表明姜黄具有广泛的药理活性,包括抗肿瘤[2]、抗炎[3]、抗氧化[4]、抗病毒[5]、免疫调节[6]等。本实验对姜黄根茎95%的乙醇提取物的正丁醇萃取部分进行分离纯化,得到13个化合物,分别鉴定为5-羟基没药酮(5-hydroxyl- bisabolon-9-one,1)、环姜黄素(cyclocurcumin,2)、环去甲氧基姜黄素(demethoxyl cyclocurcumin,3)、异环去甲氧基姜黄素(isodemethoxyl cyclocurcumin,4)、姜黄素(curcumin,5)、去氧姜黄素(demethoxyl curcumin,6)、阿魏酸甲酯(methyl ferulate,7)、香草醛(vanillin,8)、对羟基苯甲醛(4-hydroxyl benzoic aldehyde,9)、4-(4-羟基苯基)-2-丁酮 [4-(4-hydroxyl phenyl)-2-butanone,10]、4-(4-羟基-3-甲氧基苯基)-2-丁酮 [4-(4-hydroxyl-3-methoxyl phenyl)-2-butanone,11]、4-(4-羟基苯基)-3-丁烯-2-酮[4-(4-hydroxyl phenyl)-3-buten-2-one,12]、4-(4-羟基-3-甲氧基苯基)-3-丁烯-2-酮[4-(4-hydroxyl-3- methoxyl phenyl)-3-buten-2-one,13]。其中化合物1为未见文献报道的新化合物,命名为5-羟基没药酮;化合物4为首次从该植物中分离得到。
1 仪器与材料Bruker-ARX-400和Bruker-AV-600型核磁共振仪(TMS内标,瑞士Bruker公司);Shimadzu LC-10A分析型高效液相色谱仪和Shimadzu LC-8A型制备型高效液相色谱仪配Shimadzu SPD-10AT型紫外检测器(日本Shimadzu公司);Perkin-Elmer 241MC旋光仪;Bruker IFS-55红外光谱仪;Micro TOF-Q质谱仪(美国Bruker公司)。薄层色谱用硅胶和柱色谱用硅胶(青岛海洋化工有限公司);Sephadex LH-20(瑞士Pharmacia公司);普通色谱用分析纯试剂和高效液相用色谱纯试剂(山东禹王化工有限公司);氘代试剂(瑞士ARMAR公司)。
药材姜黄购于安徽省亳州市济人药业有限公司,经沈阳药科大学路金才教授鉴定为姜黄Curcuma longa L. 的干燥根茎,标本(20110829)保存于沈阳药科大学天然药物化学教研室。
2 提取与分离8 kg的干燥姜黄根茎切片,粉碎,用95%乙醇加热回流提取,得到浸膏约500 g。将浸膏分散在水中,依次用石油醚、醋酸乙酯、正丁醇连续萃取,将各部分萃取液浓缩,分别得到石油醚萃取物约150 g(明显的油状物),醋酸乙酯浸膏350 g,正丁醇浸膏65 g。其中正丁醇萃取物经硅胶柱色谱,石油醚-丙酮(100:0→1:1)梯度洗脱,得到8个馏份Fr. 1~8。Fr. 1经硅胶柱色谱,石油醚-丙酮(100:0→1:1)梯度洗脱,得到3个馏份Fr. 1.1~1.3,Fr. 1.3经过Sephadex LH-20凝胶柱色谱洗脱,制备高效液相色谱洗脱得到化合物1(1.2 mg);Fr. 3经过硅胶柱色谱二氯甲烷-甲醇(100:0→1:1)梯度洗脱,得到3个馏份Fr. 3.1~3.3,Fr. 3.2经过Sephadex LH-20凝胶柱色谱洗脱,制备高效液相色谱洗脱得到化合物7(3.2 mg)、8(2.2 mg)、9(2.6 mg),Fr. 3.3经制备高效液相色谱洗脱得到化合物10(2.6 mg)、11(5.0 mg)、12(2.5 mg)、13(4.6 mg);Fr. 5中直接得到化合物5(15 g);Fr. 6经过硅胶柱色谱,二氯甲烷-甲醇(100:0→1:1)梯度洗脱,得到3个馏份Fr. 6.1~6.3,Fr. 6.1经重结晶得到得到化合物6(10.0 mg),Fr. 6.1经制备高效液相色谱洗脱得到化合物2(3.3 mg),Fr. 6.3经制备高效液相色谱洗脱得到化合物3(9.1 mg)和4(11.8 mg)。
3 结构鉴定化合物1:无色油状物(甲醇),HR-ESI-MS m/z: 273.146 3 [M+Na]+ (计算值273.146 7) 给出分子式C15H22O3。[α]20D +37.1° (c 0.01,CH3OH)。1H-NMR (600 MHz,CDCl3) δ: 6.08 (1H,s,H-10),5.78 (1H,q,J = 1.2 Hz,H-3),4.36 (1H,t,J = 5.0 Hz,H-5),2.71 (1H,m,H-7),2.51 (1H,dt,J = 10.0,5.0 Hz,H-1),2.43 (1H,dd,J = 15.0,9.0 Hz,H-8a),2.32 (1H,dt,J = 15.0,7.8 Hz,H-8b),2.14 (1H,m,H-6a),2.12 (3H,brs,H-12),2.06 (1H,dt,J = 14.1,5.0 Hz,H-6b),0.90 (3H,brs,H-15),1.87 (3H,brs,H-13),2.02 (3H,d,J = 6.6 Hz,H-14);13C-NMR (150 MHz,CDCl3) δ: 45.9 (C-1),200.3 (C-2),127.3 (C-3),158.9 (C-4),67.2 (C-5),32.2 (C-6),27.7 (C-7),48.9 (C-8),200.1 (C-9),123.7 (C-10),155.8 (C-11),27.7 (C-12),21.0 (C-13),20.8 (C-14),17.1 (C-15)。以上碳氢信号通过HSQC谱进行了全归属。与bisabolon-9-one的碳谱数据[7]相比较,有3个位置有明显的差别:C-4 (Δδ +3.6),C-5 (Δδ +37.2) 和C-6 (Δδ +9.2),说明化合物1可能是bisabolon-9-one的羟基化衍生物。HMBC谱(图 1)中,氢信号δH 6.08 (1H,s,H-10) 与δC 200.1 (C-9)、27.7 (C-12) 相关证明了α,β-不饱和酮结构片段的存在,氢信号δH 4.36 (1H,t,J = 5.0 Hz,H-5) 与δC 127.3 (C-3) 相关,δH2.14 (1H,m,H-6a) 与δC200.3 (C-2) 相关推断出α,β-不饱和环酮的结构片段,同时δH 0.90 (3H,brs,H-15) 与δC 45.9 (C-1)、48.9 (C-8) 相关确证了侧链与α,β-不饱和环酮的连接位置在1位。H-6a、H-6b对H-5的偶合使得H-5裂分为三重峰,且偶合常数为Jae = Jee = 5.0 Hz,根据偶合常数与二面角的关系说明H-5处于平伏键;H-6b对H-1偶合,J = 10.0 Hz,说明H-6b与H-1处于直立键。NOESY谱中,未观察到H-5与H-1有相关,说明H-5与H-1位于环的两侧。根据以上信息,化合物1的结构见图 2。经检索为一个未见文献报道的新化合物,命名为为5-羟基没药酮。
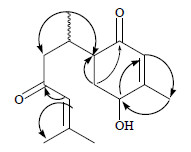 | 图 1 化合物1的HMBC相关图 Fig.1 Key HMBC correlations of compound 1 |
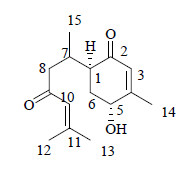 | 图 2 化合物1的化学结构式 Fig.2 Structure of compound 1 |
化合物2:淡黄色粉末状固体(甲醇),10%硫酸乙醇溶液显色剂显棕色或红色。1H-NMR (300 MHz,DMSO-d6) δ: 7.23 (1H,brs,H-2″),7.20 (1H,d,J = 15.3 Hz,H-7),7.12 (1H,d,J = 1.5 Hz,H-2′),7.05 (1H,brd,J = 8.1 Hz,H-6″),6.95 (1H,dd,J = 7.8,1.5 Hz,H-6′),6.82 (1H,d,J = 8.1 Hz,H-5″),6.81 (1H,d,J = 15.3 Hz,H-6),6.74 (1H,d,J = 7.8 Hz,H-5′),5.55 (1H,s,H-4),5.45 (1H,dd,J = 13.5,3.0 Hz,H-1),3.80 (3H,s,4′-OH),3.79 (3H,s,4″-OH),3.00 (1H,dd,J = 16.8,13.5 Hz,H-2a),2.47 (1H,dd,J = 16.8,3.0 Hz,H-2b);13C-NMR (150 MHz,DMSO-d6) δ: 80.3 (C-1),42.3 (C-2),192.3 (C-3),104.9 (C-4),168.5 (C-5),119.8 (C-6),136.9 (C-7),129.5 (C-1′),111.3 (C-2′),148.0 (C-3′),147.5 (C-4′),115.8 (C-5′),121.1 (C-6′),126.7 (C-1″),111.1 (C-2″),148.1 (C-3″),148.8 (C-4″),118.8 (C-5″),123.2 (C-6″)。以上数据与文献报道基本一致[8],故鉴定化合物2为环姜黄素。
化合物3:淡黄色粉末状固体(甲醇-水54:46),254 nm紫外光下有吸收,10%硫酸乙醇溶液显色剂显棕色或红色。1H-NMR (300 MHz,DMSO-d6) δ: 7.36 (2H,d,J = 8.4 Hz,H-2′,6′),7.23 (1H,d,J = 1.5 Hz,H-2″),7.19 (1H,d,J = 15.9 Hz,H-7),7.06 (1H,dd,J = 9.6,1.5 Hz,H-6″),6.82 (2H,d,J = 8.4 Hz,H-3′,5′),6.77 (1H,d,J = 9.6 Hz,H-5″),6.76 (1H,d,J = 15.9 Hz,H-6),5.55 (1H,s,H-4),5.46 (1H,dd,J = 14.1,3.3 Hz,H-1),3.79 (3H,s,3″-OCH3);13C-NMR (150 MHz,DMSO-d6) δ: 80.2 (C-1),42.3 (C-2),192.2 (C-3),105.0 (C-4),168.4 (C-5),119.7 (C-6),136.5 (C-7),129.5 (C-1′),128.5 (C-2′,6′),115.3 (C-3′,5′),157.8 (C-4′),122.1 (C-1″),111.1 (C-2″),148.1 (C-3″),147.9 (C-4″),115.8 (C-5″),126.7 (C-6″)。以上数据与文献报道基本一致[8],故鉴定化合物3为环去甲氧基姜黄素。
化合物4:淡黄色粉末状固体(甲醇-水54:46),254 nm紫外光下有吸收,表现出与化合物3很接近的色谱保留行为。1H-NMR (300 MHz,DMSO-d6) δ: 7.21 (1H,d,J = 16.2 Hz,H-7),7.12 (1H,d,J = 2.4 Hz,H-2′),6.95 (1H,dd,J = 8.1,2.4 Hz,H-2′),6.81 (2H,d,J = 8.1 Hz,H-3″,5″),6.81 (1H,d,J = 8.1 Hz,H-5′),6.77 (2H,d,J = 8.1 Hz,H-2″,6″),6.71 (1H,d,J = 16.2 Hz,H-6),5.56 (1H,s,H-4),5.45 (1H,dd,J = 13.5,3.3 Hz,H-1),3.80 (3H,s,4′-OCH3),2.99 (1H,dd,J = 16.8,13.5 Hz,H-2a),2.48 (1H,overlapped,H-2b);13C-NMR (150 MHz,DMSO-d6) δ: 79.9 (C-1),42.2 (C-2),192.2 (C-3),104.9 (C-4),168.4 (C-5),118.8 (C-6),136.8 (C-7),129.0 (C-1′),111.1 (C-2′),148.7 (C-3′),147.9 (C-4′),119.7 (C-5′),115.3 (C-6′),126.7 (C-1″),129.6 (C-2″,6″),159.2 (C-4″),115.7 (C-3″,5″),55.7 (4′-OCH3)。结合文献报道[9],确定化合物4为异环去甲氧基姜黄素。该化合物为首次从植物中分离得到,并首次测定了氢谱和碳谱核磁数据。
化合物5:黄色粉末(二氯甲烷),溶液有强烈的紫外吸收且有荧光。1H-NMR (300 MHz,DMSO-d6) δ: 7.56 (2H,d,J = 15.9 Hz,H-1,6),7.33 (2H,d,J = 1.5 Hz,H-2′,2″),7.16 (2H,dd,J = 8.1,1.5 Hz,H-6′,6″),6.84 (2H,d,J = 8.1 Hz,H-5′,5″),6.76 (2H,d,J = 15.9 Hz,H-2,7),6.06 (1H,s,H-4),3.83 (6H,s,3′,3″-OCH3)。以上数据与文献报道基本一致[10],故鉴定化合物5为姜黄素。
化合物6:黄色针状结晶(丙酮),10%硫酸乙醇溶液显色剂显紫红色。1H-NMR (300 MHz,DMSO-d6) δ: 10.13 (1H,s,4′-OH),9.73 (1H,s,4′-OH),7.63 (1H,d,J = 9.0 Hz,H-5′),7.61 (1H,d,J = 15.9 Hz,H-1),7.59 (1H,d,J = 13.8 Hz,H-7),7.38 (1H,brs,H-2′),7.21 (1H,brd,J = 9.0 Hz,H-6′),6.88 (4H,d,J = 8.1 Hz,H-2″,3″,5″,6″),6.83 (1H,d,J = 13.8 Hz,H-6),6.75 (1H,d,J = 15.9 Hz,H-2),6.10 (1H,s,H-4),3.89 (3H,s,3′-OCH3)。以上数据与文献报道基本一致[11],故鉴定化合物6为去甲氧基姜黄素。
化合物7:白色无定形粉末(甲醇),254 nm紫外光下有吸收。1H-NMR (600 MHz,CDCl3) δ: 7.62 (1H,d,J = 16.2 Hz,H-3),7.07 (1H,dd,J = 8.4,1.8 Hz,H-6′),7.02 (1H,d,J = 1.8 Hz,H-2′),6.92 (1H,d,J = 8.4 Hz,H-5′),6.29 (1H,d,J = 16.2 Hz,H-2),5.91 (1H,s,4′-OH),3.93 (3H,s,3′-OCH3),3.73 (3H,s,1-OCH3)。以上数据与文献报道基本一致[12],故鉴定化合物7为阿魏酸甲酯。
化合物8:白色针状结晶(甲醇),254 nm紫外光下有吸收。1H-NMR (600 MHz,CDCl3) δ: 9.28 (1H,s,-CHO) 7.43 (1H,dd,J = 8.4,1.8 Hz,H-6),7.42 (1H,d,J = 1.8 Hz,H-2),7.04 (1H,d,J = 8.4 Hz,H-5),6.35 (1H,brs,4-OH),3.96 (3H,s,3-OCH3)。以上数据与文献报道基本一致[13],与市售试剂香草醛共薄层,Rf值一致,故鉴定化合物8为香草醛。
化合物9:白色针状结晶(甲醇)。1H-NMR (300 MHz,DMSO-d6) δ: 9.78 (1H,s,-CHO),9.11 (1H,s,4-OH),7.75 (2H,d,J = 8.7 Hz,H-2,6),6.93 (2H,d,J = 8.7 Hz,H-3,5)。以上数据与文献报道基本一致[14],故鉴定化合物9为对羟基苯甲醛。
化合物10:白色无定形粉末(甲醇),254 nm紫外灯下有暗斑。1H-NMR (300 MHz,CDCl3) δ: 7.05 (2H,d,J = 8.4 Hz,H-2′,6′),6.75 (2H,d,J = 8.4 Hz,H-3′,5′),2.83 (2H,m,H-4),2.73 (2H,m,H-3),2.13 (3H,s,H-1)。以上数据与文献报道基本一致[15],故鉴定化合物10是4-(4-羟基苯基)-2-丁酮。
化合物11:白色无定形粉末(甲醇),254 nm紫外灯下有暗斑。1H-NMR (300 MHz,CDCl3) δ: 6.82 (1H,d,J = 8.1 Hz,H-5′),6.69 (1H,d,J = 1.8 Hz,H-2′),6.66 (1H,dd,J = 8.1,1.8 Hz,H-6′),3.87 (3H,s,3′-OCH3),2.83 (2H,m,H-4),2.72 (2H,m,H-3),2.14 (3H,s,H-1)。以上数据与文献报道基本一致[16],故鉴定化合物11为4-(4-羟基-3-甲氧基苯基)-2-丁酮。
化合物12:白色针状结晶(甲醇),254 nm紫外灯下有暗斑。1H-NMR (300 MHz,CDCl3) δ: 7.48 (1H,d,J = 16.2 Hz,H-3),7.46 (2H,d,J = 8.7 Hz,H-2′,6′),6.87 (2H,d,J = 8.7 Hz,H-3′,5′),6.62 (1H,d,J = 16.2 Hz,H-4),2.38 (3H,s,H-1)。以上数据与文献报道基本一致[17],故鉴定化合物12为4-(4-羟基苯基)-3-丁烯-2-酮。
化合物13:淡黄色针状结晶(甲醇),254 nm紫外灯下有暗斑。1H-NMR (300 MHz,CDCl3) δ: 7.45 (1H,d,J = 16.2 Hz,H-4),7.09 (1H,dd,J = 1.8,8.1 Hz,H-6′),7.07 (1H,d,J = 1.8 Hz,H-2′),6.93 (1H,d,J = 8.1 Hz,H-5′),6.59 (1H,d,J = 16.2 Hz,H-3),6.03 (1H,brs,3′-OH),3.92 (3H,s,4′-OCH3),2.36 (3H,s,H-1)。以上数据与文献报道基本一致[17],故鉴定化合物13为4-(4-羟基-3-甲氧基苯基)-3-丁烯-2-酮。
| [1] | Li S Y, Yuan W, Deng G R, et al. Chemical composition and product quality control of turmeric(Curcuma longa L.)[J]. Pharm Crops, 2011, 5(1):28-54. |
| [2] | Wagner H K, Wolff P M. Natural Products and Plant Drugs with Pharmacological Biological or Therapeutical Activity[M]. New York:Springer Berlin Heidelberg, 1977. |
| [3] | Jurenka J S. Anti-inflammatory properties of Curcumin, a major constituent of Curcuma longa:a review of preclinical and clinical research[J]. Altern Med Rev, 2009, 14(2):141-153. |
| [4] | Sharma O P. Antioxidant activity of curcumin and related compounds[J]. Biochem Pharm, 1976, 25(15):1811-1812. |
| [5] | Nunziatina D T, Cosimo P, Cinzia C, et al. Structure and in vitro antiviral activity of sesquiterpene glycosides from Calendula arvensis[J]. J Nat Prod, 1990, 53(4):830-835. |
| [6] | Arnason J T, Isman M B, Philogene B J R, et al. Mode of action of the sesquiterpene lactone, tenulin, from Helenium amarum against herbivorous insects[J]. J Nat Prod, 1987, 50(4):690-695. |
| [7] | 曾永篪, 梁键谋, 曲戈霞, 等. 姜黄的化学成分研究I:没药烷型倍半萜[J]. 中国药物化学杂志, 2007, 17(4):238-242. |
| [8] | Kiuchi F, Goto Y, Sugimoto N, et al. Nematocidal activity of turmeric:synergistic action of curcuminoids[J]. Chem Pharm Bull, 1993, 41(9):1640-1643. |
| [9] | Jiang J L, Jin X L, Zhang H, et al. Identification of antitumor constituents in curcuminoids from Curcuma longa L. based on the composition-activity relationship[J]. J Pharm Biomed Anal, 2012, 70(11):664-670. |
| [10] | Ragasa C Y, Laguardiaand M A, Rideout J A, et al. Antimicrobial sesquiterpenoids and diarylheptanoid from Curcuma domestica[J]. Chem Res Commun, 2005, 18(1):21-24. |
| [11] | Kiuchi F, Goto Y, Suqimoto N, et al. Nematocidal activity of turmeric:synergistic action of curcuminoids[J]. Chem Pharm Bull, 1993, 41(9):1640-1643. |
| [12] | Kwon Y S, Kim C M. Antioxidant constituents from the stem of Sorghum bicolor[J]. Arch Pharm Res, 2003, 26(7):535-539. |
| [13] | Vladimir P P, Dusica S, Sladjana B N, et al. Structural characterization of some vanilic Mannich bases:Experimental and theoretical study[J]. J Mol Struct, 2015, 1098(10):34-40. |
| [14] | Vatcharin R, Nanthaphong K, Yaowapa S, et al. Cyclohexene, diketopiperazine, lactone and phenol derivatives from the sea fan-derived fungi Nigrospora sp. PSU-F11 and PSU-F12[J]. Arch Pharm Res, 2010, 33(3):375-380. |
| [15] | Ayer W A, Singer P P. Phenolic metabolites of the bird's nest fungus Nidula niveo-tomentosa[J]. Phytochemistry, 1980, 19(12):2717-2721. |
| [16] | Kumar V, Sharma A, Sinha A K, et al. Solid-supported green synthesis of substituted hydrocinnamic esters by focused microwave irradiation[J]. Helv Chim Acta, 2006, 89(3):483-495. |
| [17] | Takao K,Bunta W,Shiro S,et al.Characterization of raspberry ketone/zingerone synthase,catalyzing the alpha,beta-hydrogenation of phenylbutenones in raspberry fruits[J].Biochem Bioph Res Co,2011,412(1): 104-108. |
 2016, Vol. 47
2016, Vol. 47


