辽东楤木Aralia elata (Miq.) Seem. 又名龙牙楤木,俗称刺嫩芽,为五加科(Araliaceae)楤木属Aralia L. 植物,在我国民间地区常作为药食同源的药用植物用于心肌梗死、神经衰弱等症的治疗[1]。前期研究发现,辽东楤木中的三萜皂苷类成分具有治疗冠心病心绞痛的作用[2, 3, 4],目前以楤木皂苷为主要成分的楤木心脉通胶囊已获得药物临床研究批件并完成了临床观察。在此基础上,课题组深入研究发现楤木皂苷中的活性单体化合物齐墩果酸-3- O-β-D-吡喃葡萄糖醛酸表现出了较好的心肌保护作用[5, 6, 7],但其作用机制、构效关系的深入阐述仍不明确。同时目前国内外对齐墩果烷型皂苷的生物学活性研究主要集中于抗肿瘤和抗骨质疏松等方面,抗心肌缺血、心肌保护活性等活性研究报道较少[8, 9, 10, 11]。鉴于天然产物结构改造是开发新药的重要途径,且目前齐墩果酸-3-O-β-D-吡喃葡萄糖醛酸类衍生物的心肌保护活性仍未被报道,因此,本实验基于该类辽东楤木单体化合物,通过结构修饰设计合成一系列结构衍生物F1~F10,包括将糖给体、苷元以及酰胺基团的替换等,以考察原型化合物的构效关系,以期进一步发现心肌保护活性良好的化合物,为中药新药的研究与开发奠定实验基础。
1 仪器与试药艾卡(IKA®)C-MAG HS 7型磁力搅拌器,艾卡(IKA®)RV10 basic型旋转蒸发仪,梅特勒-托利多(Mettler Toledo AL104)电子天平,Bruker Avance III 600型核磁共振波谱仪,赛默飞世尔(Thermofisher)LTQ-Obitrap XL液质联用仪,IR谱采用Analect RFX-65A型红外光谱仪测定(KBr压片法),BYLABUV-III紫外灯(北京炳洋科技有限公司),37 ℃、5% CO2恒温培养箱(日本Sanoy公司);IX51倒置荧光显微镜(OLYMPUS 公司),KC junior微孔板分光光度计(BIOTEK公司)。
D-葡萄糖(质量分数99.5%)、D-半乳糖(质量分数99.5%)均购自国药集团化学试剂有限公司;齐墩果酸(质量分数>98%,绵阳东方源生物科技有限公司);熊果酸(质量分数>98%,长沙麓园生物科技有限公司)。溴苄(BnBr)、1-羟基苯并三氮唑(HOBt)、1-乙基-(3-二甲基氨基丙基) 碳化二亚胺盐酸盐(EDCI)、AMBERLITE IR-120(阳离子交换树脂)以及各种胺等均购于Energy Chemical公司,常规试剂市售分析纯和化学纯,柱色谱用硅胶及薄层色谱用硅胶G、H、GF254和柱色谱用硅胶均购于青岛海洋化工有限公司。大鼠心肌细胞H9c2购于中国科学院细胞库。
2 方法 2.1 目标化合物的设计辽东楤木有效单体成分齐墩果酸-3-O-β-D-吡喃葡萄糖醛酸的化学结构由两部分组成,葡萄糖醛酸部分和齐墩果酸苷元部分通过3位苷键相连。本实验设计的目标化合物为图 1中的化合物F1~F10,通过糖给体、苷元以及酰胺基团的替换,以考察原型化合物的构效关系。其中,化合物F1~F7为齐墩果酸苷元不变,葡萄糖给体替换葡萄糖醛酸给体,苷元28位羧基分别形成不同的酰胺,取代基R5分别为正丙基(F1)、环己基(F2)、苄基(F3)、N-甲基哌嗪基(F4)、吗啉基(F5)、正癸基(F6)、对甲氧基苯基(F7);化合物F8~F10为熊果酸苷元替换齐墩果酸苷元,半乳糖给体替换葡萄糖醛酸给体,苷元28位羧基分别形成不同的酰胺,取代基R5分别为正丙基(F8)、环己基(F9)、苄基(F10)。
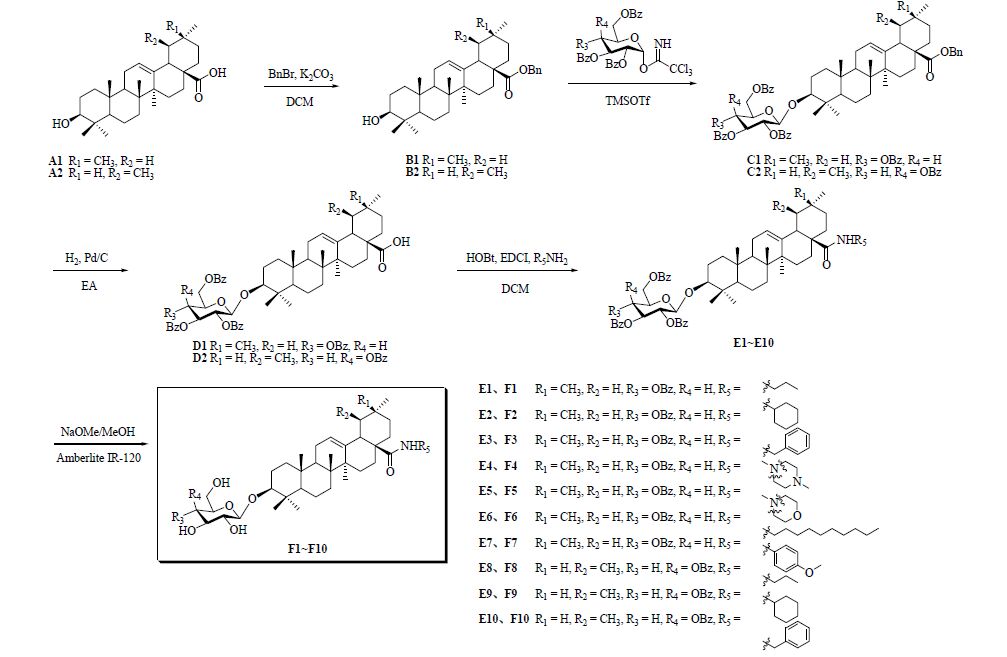
|
图 1 辽东楤木单体衍生物的合成路线 Fig. 1 Synthetic routes of monomeric derivatives from A. elata |
本实验对衍生物的合成采用一条5步反应的路线,见图 1。分别以齐墩果酸A1和熊果酸A2为起始原料,经苄基保护(B1~B2)、Lewis酸催化糖苷化(C1~C2)、催化氢化脱苄基保护(D1~D2)、酰胺化(E1~E10)以及脱去糖给体上的苯甲酰基保护,最终得到目标化合物F1~F10。
2.3 合成方法 2.3.1 糖给体a和b的合成参考文献的合成方法[12, 13],以葡萄糖和半乳糖为原料,采用经典的Schmidit法分别制备得到葡萄糖三氯乙酰亚胺酯给体(a,收率76%)和半乳糖三氯乙酰亚胺酯给体(b,收率73%)。
2.3.2 中间体B1和B2的合成分别于500 mL圆底烧瓶中准确称取齐墩果酸A1或熊果酸A2(10.0 g,21.8 mmol),加入300 mL二氯甲烷。依次加入四丁基溴化铵(0.8 g,2.5 mmol)以及碳酸钾(7.4 g,53.6 mmol)的50 mL水溶液,搅拌均匀。冰浴下滴加溴苄(3.2 mL,26.8 mmol),滴毕升温至室温,过夜反应。反应结束后分液,有机相依次用0.1 mol/L稀盐酸、饱和碳酸氢钠水溶液、饱和氯化钠水溶液洗涤,合并有机层,无水硫酸镁干燥,抽滤,滤液浓缩,残余物通过硅胶柱色谱纯化,石油醚-醋酸乙酯(8∶1)洗脱分别得到白色固体B1和B2。B1:10.8 g,收率91%,HR-ESI-MS m/z: 569.397 0 [M+Na]+;B2:11.1 g,收率93%,HR-ESI-MS m/z: 569.397 4 [M+Na]+。
2.3.3 中间体C1和C2的合成分别于100 mL圆底烧瓶中准确称取中间体B1(3.3 g,6.0 mmol)和a(5.8 g,7.9 mmol),或中间体B2(3.3 g,6.0 mmol)与b(5.8 g,7.9 mmol),加入50 mL无水二氯甲烷溶解,加入0.5 g 粉末状分子筛,在氮气保护下室温搅拌反应1 h,随后加入催化量的Le wis酸(TMSOTf,60 μL,0.3 mol)继续反应2 h,直至TLC监测反应结束,展开剂为石油醚-醋酸乙酯(8∶1)。加入1.0 mL三乙胺淬灭反应。滤过除掉分子筛,滤液浓缩,残余物通过硅胶柱色谱纯化,石油醚-醋酸乙酯(10∶1)洗脱分别得到白色固体C1和C2。C1:5.4 g,收率80%,HR-ESI-MS m/z: 1 147.555 0 [M+Na]+;C2:5.8 g,收率85%,HR-ESI-MS m/z: 1 147.554 8 [M+Na]+。
2.3.4 中间体D1和D2的合成分别于100 mL圆底烧瓶中准确称取中间体C1或C2(3.0 g,2.6 mmol),加入30 mL醋酸乙酯溶解,预通氮气3 min后,加入10% Pd/C催化剂1.5 mg,随后开始通氢气,外浴80 ℃下缓慢升温至回流反应3 h,直至TLC监测反应结束,展开剂为石油醚-醋酸乙酯(4∶1)。滤过除掉钯碳,滤液浓缩,残余物通过硅胶柱色谱纯化,石油醚-醋酸乙酯(3∶1)洗脱分别得到白色固体D1和D2。D1:2.6 g,收率97%,HR-ESI-MS m/z: 1 057.507 7 [M+Na]+;D2:2.5 g,收率92%,HR-ESI-MS m/z: 1 057.508 1 [M+Na]+。
2.3.5 中间体E1~E10和目标化合物F1~F10的合成分别于50 mL圆底烧瓶中准确称取中间体D1或D2(1.0 g,0.98 mmol),加入15 mL无水二氯甲烷溶解,随后加入HOBt(0.2 g,1.46 mmol)和EDCI(0.28 g,1.46 mmol),室温下搅拌反应1 h后,冰浴下缓慢加入各种胺R5NH2(3.92 mmol),升至室温反应4~16 h,直至TLC监测反应结束,展开剂为二氯甲烷-甲醇(100∶1)。后处理时反应液依次用0.1 mol/L稀盐酸、饱和碳酸氢钠水溶液、饱和氯化钠水溶液洗涤,无水硫酸钠干燥过夜。滤过,滤液浓缩,得中间体E1~E10粗品。中间体E1~E10不经纯化,分别溶于适量的干燥甲醇-二氯甲烷(3∶1)溶液中,加入1 mol/L甲醇钠甲醇溶液1.6 mL,室温下反应6 h,TLC监测反应结束,展开剂为二氯甲烷-甲醇(10∶1)。加入阳离子交换树脂Amberlite IR-120调节pH值至中性,抽滤,滤液浓缩。残余物通过硅胶柱色谱纯化,二氯甲烷-甲醇(15∶1)洗脱得到目标化合物F1~F10。辽东楤木单体衍生物的合成反应路线见图 1。
2.4 衍生物体外心肌细胞保护活性研究大鼠胚胎期心脏的H9c2心肌细胞,在添加胎牛血清的达尔贝克改良的Eagle’s(DMEM)培养基中,于37 ℃下5% CO2的条件下培养。当细胞生长至融合80%~90%时,在相同培养条件下将细胞进行分组处理。包括对照组(实验全过程中使用无血清培养液处理)、H2O2模型组(无血清培养液培养6 h后,加入终浓度为150 μmol/L H2O2作用6 h)、试验组(分别将含有相应浓度的各目标化合物F1~F10的无血清培养液作用6 h后,弃去含药培养液加入终浓度为150 μmol/L H2O2作用6 h)。取对数生长期的细胞以1×104个/孔的密度接种于96孔板中,在5% CO2、37 ℃条件培养36 h后进行处理。处理结束后,每孔加入5 mg/mL MTT溶液20 μL,37 ℃孵育4 h后小心移除培养液,每孔中加入100 μL DMSO,微孔板震荡器震荡10 min使甲臜结晶充分溶解后,在微孔板扫描酶标仪测560 nm处吸光度(A)值。将各测试孔的A值减去本底A值(无血清培养基加MTT,无细胞),根据各孔A值计算平均值。
细胞存活率=(A加药细胞-A本底)/(A对照细胞-A本底)
3 结果与讨论 3.1 目标化合物的结构鉴定 化合物F1:白色粉末。收率67%。HR-ESI-MS m/z: 682.465 8 [M+Na]+。1H-NMR (600 MHz,C5D5N) δ: 7.30 (1H,t,J = 5.6 Hz,N-H),5.46 (1H,t,J = 3.3 Hz,H-12),4.96 (1H,d,J = 7.9 Hz,H-1′),4.60 (1H,dd,J = 11.6,2.5 Hz,H-3′),4.43 (1H,dd,J = 11.6,5.3 Hz,H-2′),4.29~4.23 (2H,m,H-4′,5′),4.08~4.02 (2H,m,H-6′),3.52~3.46 (1H,m,H-31a),3.42 (1H,dd,J = 11.8,4.7 Hz,H-3),3.31~3.25 (1H,m,H-31b),3.11 (1H,d,J = 11.8 Hz,H-18),1.35 (3H,s,CH3),1.30 (3H,s,CH3),1.04 (3H,s,CH3),0.97 (3H,s,CH3),0.95 (3H,s,CH3),0.94 (3H,s,CH3),0.90 (3H,s,CH3),0.87 (3H,t,J = 7.3 Hz,H-33);13C-NMR (150 MHz,C5D5N) δ: 177.4,145.0,122.7,106.8,88.9,78.8,78.2,75.9,71.9,63.1,55.8,48.0,46.8,46.5,42.3,42.1,39.8,39.6,39.5,38.8,37.0,34.4,
33.8,33.2,33.2,32.2,30.9,28.3,28.0,26.6,26.2,23.9,23.8,23.7,18.5,17.5,17.1,15.5。
化合物F2:白色粉末。收率62%。HR-ESI-MS m/z: 722.497 4 [M+Na]+。1H-NMR (600 MHz,C5D5N) δ: 6.66 (1H,d,J = 7.6 Hz,N-H),5.48 (1H,t,J = 3.4 Hz,H-12),4.96 (1H,d,J = 7.8 Hz,H-1′),4.61 (1H,dd,J = 11.6,2.6 Hz,H-3′),4.48~4.40 (2H,m,H-31,H-2′),4.29~4.23 (2H,m,H-4′,5′),4.08~4.02 (2H,m,H-6′),3.41 (1H,dd,J = 11.8,4.3 Hz,H-3),3.05 (1H,dd,J = 13.4,4.0 Hz,H-18),1.35 (3H,s,CH3),1.30 (3H,s,CH3),1.05 (3H,s,CH3),0.99 (3H,s,CH3),0.94 (3H,s,CH3),0.93 (3H,s,CH3),0.92 (3H,s,CH3);13C-NMR (150 MHz,C5D5N) δ: 177.5,145.0,122.7,106.8,88.9,78.8,78.3,75.9,71.9,63.1,55.8,51.5,48.0,46.8,46.2,42.3,42.1,39.8,39.5,38.8,37.0,34.4,
33.6,33.6,33.2,33.2,32.6,30.8,28.2,27.8,26.6,26.1,24.2,24.1,24.0,23.9,23.7,23.7,18.5,17.6,17.0,15.5。
化合物F3:白色粉末。收率68%。HR-ESI-MS m/z: 730.466 3 [M+Na]+。1H-NMR (600 MHz,C5D5N) δ: 8.00 (1H,t,J = 5.8 Hz,N-H),7.50~7.49 (2H,m,Ph-H-2″,6″),7.37~7.34 (2H,m,Ph-H-3″,5″),7.28~7.25 (1H,m,Ph-H-4″),5.44 (1H,t,J = 3.3 Hz,H-12),4.96 (1H,d,J = 7.7 Hz,H-1′),4.79 (1H,dd,J = 15.0,6.0 Hz,H-31a),4.63~4.59 (2H,m,H-31b,H-3′),4.43 (1H,dd,J = 11.6,5.4 Hz,H-2′),4.29~4.23 (2H,m,H-4′,5′),4.08~4.02 (2H,m,H-6′),3.41 (1H,dd,J = 11.8,4.3 Hz,H-3),3.18 (1H,dd,J = 13.3,4.1 Hz,H-18),1.34 (3H,s,CH3),1.30 (3H,s,CH3),1.03 (3H,s,CH3),0.93 (6H,s,2×CH3),0.89 (3H,s,CH3),0.88 (3H,s,CH3);13C-NMR (150 MHz,C5D5
N) δ: 177.5,144.9,140.7,128.7,128.1,127.2,122.8,106.9,88.8,78.8,78.3,75.8,71.9,63.1,55.8,48.0,46.8,46.5,43.5,42.2,41.9,
39.8,39.5,38.7,37.0,34.4,33.8,33.2,33.1,30.9,28.3,27.9,26.6,26.1,23.8,23.7,23.3,18.5,17.4,17.0,15.5。
化合物F4:白色粉末。收率47%。HR-ESI-MS m/z: 723.492 6 [M+Na]+。1H-NMR (600 MHz,C5D5N) δ: 5.44 (1H,t,J = 3.5 Hz,H-12),4.95 (1H,d,J = 7.7 Hz,H-1′),4.59 (1H,dd,J = 11.7,2.4 Hz,H-3′),4.42 (1H,dd,J = 11.6,5.3 Hz,H-2′),4.28~4.21 (2H,m,H-4′,5′),4.06~4.00 (2H,m,H-6′),3.77~3.36 (4H,m,H-31,32),3.43~3.40 (2H,m,H-3,18),2.38~2.34 (4H,m,H-33,34),2.21 (3H,s,CH3),1.35 (3H,s,CH3),1.30 (3H,s,CH3),1.04 (3H,s,CH3),0.98 (3H,s,CH3),0.96 (3H,s,CH3),0.95 (3H,s,CH3),0.90 (3H,s,CH3);13C-NMR (150 MHz,C5D5N) δ: 174.9,145.5,121.9,106.9,88.9,78.8,78.3,75.9,71.9,63.1,56.0,55.6,48.2,47.6,46.8,46.8,46.0,45.7,44.2,42.3,39.7,39.6,
38.8,37.1,34.3,33.4,33.2,30.4,30.2,28.4,28.3,26.6,26.2,24.2,23.8,23.8,22.9,18.6,17.4,17.1,15.6。
化合物F5:白色粉末。收率51%。HR-ESI-MS m/z: 710.461 0 [M+Na]+。1H-NMR (600 MHz,C5D5N) δ: 5.44 (1H,t,J = 3.3 Hz,H-12),4.95 (1H,d,J = 7.8 Hz,H-1′),4.59 (1H,dd,J = 11.7,2.5 Hz,H-3′),4.42 (1H,dd,J = 11.6,5.4 Hz,H-2′),4.28~4.21 (2H,m,H-4′,5′),4.07~4.00 (2H,m,H-6′),3.78~3.68 (8H,m,H-31~34),3.44~3.40 (2H,m,H-3,18),1.36 (3H,s,CH3),1.29 (3H,s,CH3),1.04 (3H,s,CH3),0.97 (3H,s,CH3),0.96 (3H,s,CH3),0.93 (3H,s,CH3),0.90 (3H,s,CH3);13C-NMR (150 MHz,C5D5N) δ: 175.1,145.3,122.0,106.9,88.9,78.8,78.3,75.9,71.9,67.2,63.1,56.0,48.1,47.7,46.7,46.4,44.1,42.3,39.7,39.6,38.8,37.1,
34.2,33.4,33.2,33.2,30.6,30.2,28.3,28.3,26.6,26.2,24.2,23.8,22.8,18.6,17.3,17.1,15.6。
化合物F6:白色粉末。收率44%。HR-ESI-MS m/z: 780.575 1 [M+Na]+。1H-NMR (600 MHz,C5D5N) δ: 7.26 (1H,t,J = 5.6 Hz,N-H),5.48 (1H,t,J = 3.3 Hz,H-12),4.95 (1H,d,J = 7.7 Hz,H-1′),4.59 (1H,dd,J = 11.6,2.4 Hz,H-3′),4.42 (1H,dd,J = 11.7,5.4 Hz,H-2′),4.28~4.21 (2H,m,H-4′,5′),4.07~4.00 (2H,m,H-6′),3.59~3.53 (1H,m,H-31a),3.44~3.33 (2H,m,H-3,H-31b),3.11 (1H,dd,J = 13.5,4.2 Hz,H-18),1.35 (3H,s,CH3),1.31 (3H,s,CH3),1.04 (3H,s,CH3),1.01 (3H,s,CH3),0.97 (3H,s,CH3),0.94 (3H,s,CH3),0.93 (3H,s,CH3),0.85 (3H,t,J = 7.1 Hz,H-40);13C-NMR (150 MHz,C5D5N) δ: 177.4,145.1,122.8,106.9,88.9,78.8,78.3,75.9,71.9,63.1,55.8,48.0,46.9,46.5,42.3,42.0,40.0,39.9,39.6,38.8,37.0,34.5,
33.9,33.2,33.1,32.0,30.9,30.2,29.6,29.6,28.3,28.0,27.6,26.6,26.2,23.9,23.8,
23.8,22.9,18.5,17.6,17.1,15.6,14.2。
化合物F7:白色粉末。收率61%。HR-ESI-MS m/z: 746.460 7 [M+Na]+。1H-NMR (600 MHz,C5D5N) δ: 9.01 (1H,s,N-H),7.91~7.89 (2H,m,Ph-H-2″,6″),7.02~7.00 (2H,m,Ph-H-3″,5″),5.60 (1H,t,J = 3.3 Hz,H-12),4.94 (1H,d,J = 7.8 Hz,H-1′),4.60~4.58 (1H,m,H-3′),4.43~4.40 (1H,m,H-2′),4.27~4.21 (2H,m,H-4′,5′),4.05~4.01 (2H,m,H-6′),3.64 (3H,s,CH3),3.41 (1H,dd,J = 11.8,4.4 Hz,H-3),3.24 (1H,dd,J = 13.4,4.0 Hz,H-18),1.33 (3H,s,CH3),1.31 (3H,s,CH3),1.01 (3H,s,CH3),0.95 (3H,s,CH3),0.93 (6H,s,2×CH3),0.85 (3H,s,CH3);13C-NMR (150 MHz,C5D5N) δ: 176.1,156.6,145.0,133.2,123.1,114.4,106.9,88.9,78.8,78.3,75.8,71.9,
63.1,55.8,55.3,48.0,47.3,46.8,42.3,42.0,39.9,39.5,38.8,37.0,34.4,33.3,33.2,33.0,30.9,28.3,28.0,26.6,26.2,23.9,23.8,
23.8,18.5,17.5,17.0,15.5。
化合物F8:白色粉末。收率61%。HR-ESI-MS m/z: 682.466 1 [M+Na]+。1H-NMR (600 MHz,C5D5N) δ: 7.19 (1H,t,J = 5.5 Hz,N-H),5.46 (1H,t,J = 3.3 Hz,H-12),4.88 (1H,d,J = 7.6 Hz,H-1′),4.61~4.60 (1H,m,H-3′),4.52~4.46 (3H,m,H-2′,4′,5′),4.19 (1H,dd,J = 3.4,9.6 Hz,H-6′a),4.14 (1H,t,J = 5.9 Hz,H-6′b),3.44~3.40 (2H,m,H-31a,H-3),3.33~3.28 (1H,m,H-31b),2.39 (1H,d,J = 10.7 Hz,H-18),1.35 (3H,s,CH3),1.23 (3H,s,CH3),1.03 (3H,s,CH3),0.98~0.95 (9H,m,3×CH3),0.91 (3H,s,CH3),0.87 (3H,t,J = 7.3 Hz,H-33);13C-NMR (150 MHz,C5D5N) δ: 177.5,139.8,125.7,107.4,88.7,76.8,75.3,73.2,70.2,62.5,55.9,53.5,47.9,47.8,42.6,40.1,39.9,39.6,39.6,39.3,38.9,38.3,
36.9,33.3,32.1,31.2,28.3,28
.3,26.8,24.9,23.8,23.6,23.5,18.3,17.5,17.1,15.6。
化合物F9:白色粉末。收率67%。HR-ESI-MS m/z: 722.497 2 [M+Na]+。1H-NMR (600 MHz,C5D5N) δ: 6.70 (1H,d,J = 7.4 Hz,N-H),5.49 (1H,t,J = 3.5 Hz,H-12),4.88 (1H,d,J = 7.6 Hz,H-1′),4.61~4.60 (1H,m,H-3′),4.52~4.46 (3H,m,H-2′,4′,5′),4.19 (1H,dd,J = 3.4,9.6 Hz,H-6′a),4.14 (1H,t,J = 5.9 Hz,H-6′b),4.07~4.06 (1H,m,H-31),2.38 (1H,d,J = 10.7 Hz,H-18),1.34 (3H,s,CH3),1.24 (3H,s,CH3),1.02 (3H,s,CH3),0.97~0.95 (6H,m,2×CH3),0.91 (3H,s,CH3),0.89 (3H,s,CH3);13C-NMR (150 MHz,C5D5N) δ: 177.4,139.8,125.6,107.3,88.7,76.7,75.2,73.2,70.1,62.4,55.9,53.5,51.4,48.0,47.8,42.5,39.9,39.9,39.5,39.3,38.8,38.2,
36.8,33.6,33.4,32.6,31.2,28.2,28.2,26.7,24.9,24.2,24.1,24.0,23.9,23.7,23.5,18.5,17.6,17.0,15.5。
化合物F10:白色粉末。收率65%。HR-ESI-MS m/z: 730.465 5 [M+Na]+。1H-NMR (600 MHz,C5D5N) δ: 7.78 (1H,t,J = 5.8 Hz,N-H),7.49~7.48 (2H,m,Ph-H-2″,6″),7.38~7.35 (2H,m,Ph-H-3″,5″),7.29~7.28 (1H,m,Ph-H-4″),5.43 (1H,t,J = 3.3 Hz,H-12),4.89 (1H,d,J = 7.6 Hz,H-1′),4.72 (1H,dd,J = 14.7,5.5 Hz,H-31a),4.64 (1H,dd,J = 14.8,5.7 Hz,H-31b),4.59~4.60 (1H,m,H-3′),4.50~4.46 (3H,m,H-2′,4′,5′),4.19 (1H,dd,J = 3.4,9.6 Hz,H-6′a),4.14 (1H,t,J = 5.9 Hz,H-6′b),3.43 (1H,dd,J = 11.7,4.2 Hz,H-3),2.44 (1H,d,J = 10.7 Hz,H-18),1.34 (3H,s,CH3),1.24 (3H,s,CH3),1.00 (3H,s,CH3),0.97 (3H,d,J = 6.5 Hz,CH3),0.95 (3H,s,CH3),0.91 (3H,s,CH3),0.89 (3H,s,CH3);13C-NMR (150 MHz,C5D5N) δ: 177.3,140.5,139.6,128.7,128.2,127.2,125.9,107.5,88.8,76.8,7
5.4,73.2,70.3,62.5,55.9,53.6,47.9,47.8,43.6,42.5,40.0,39.8,39.5,39.3,38.9,38.1,36.9,33.4,31.2,28.3,28.3,26.7,24.9,23.8,
23.6,21.3,18.4,17.4,17.4,17.0,15.6。
以原型化合物齐墩果酸-3-O-β-D-吡喃葡萄糖醛酸为阳性对照,在低、中、高(0.1、0.3、1.0 μg/mL)3个剂量浓度下,通过细胞学实验考察了结构修饰物F1~F10对H9c2心肌细胞的保护作用。具体结果见表 1。
|
|
表 1 辽东楤木单体衍生物的心肌保护活性结果 Table 1 Myocardial protection activity of monomeric derivatives from A. elata |
药理实验结果表明,10个衍生物在3个质量浓度下均呈现出不同程度的心肌细胞保护活性,其中化合物F3与原型化合物齐墩果酸-3-O-β-D-吡喃葡萄糖醛酸相比活性相当,尤其在低、中剂量组(0.1和0.3 μg/mL)活性更有优势。初步的构效关系研究表明:1)齐墩果酸-3-O-β-D-吡喃葡萄糖醛酸发挥心肌保护作用,结构中28位羧基并非为结构必需基团,羧基成酰胺后对活性影响较大,连有苄胺的衍生物活性较好,如化合物F3和F10,而连有较长脂肪胺和芳香胺的衍生物活性较弱,如化合物F6和F7;2)葡萄糖醛酸替换为葡萄糖或半乳糖后,对心肌保护活性影响不大,如化合物F3和F10;3)齐墩果酸苷元替换为熊果酸苷元后,对心肌保护活性影响也不大,同样如化合物F3和F10。所合成的目标化合物均为未见文献报道的化合物,为深入开展楤木单体心肌保护活性研究奠定基础,具有深入研究的 价值。
| [1] | 《全国中草药汇编》编写组. 全国中草药汇编(上册)[M]. 第2版. 北京:人民卫生出版社, 1996. |
| [2] | 孙桂波, 李锐, 周丽玲, 等. 龙牙楤木化学成分与药理作用的研究进展[J]. 中国新药与临床药理, 2003, 14(2):139-141. |
| [3] | 孙桂波, 徐惠波, 温富春, 等. 龙牙楤木总皂苷对缺氧再给氧心肌细胞损伤的保护作用[J]. 中国药理学通报, 2006, 22(9):1092-1094. |
| [4] | Kochetkoy H K. Chemical constituent of Aralia elata[J]. Dokl Akad Nauk, 1963, 50(6):1289. |
| [5] | 孙桂波, 徐惠波, 温富春, 等. 去葡萄糖竹节参皂苷IVa对心肌电生理特性的影响[J]. 中国药理学通报, 2006, 22(11):1407-1408. |
| [6] | 张家鑫, 田瑜, 钟晓明, 等. 辽东楤木三萜皂苷类化学成分的研究[J]. 中国药房, 2013, 24(15):1380-1382. |
| [7] | 孙桂波, 徐惠波, 温富春, 等. 去葡萄糖竹节参皂苷IVa抗实验性心律失常作用[J]. 中国药理学与毒理学杂志, 2006, 20(5):377-380. |
| [8] | 崔现军, 李明, 鲁卫星. 龙牙楤木总皂苷对大鼠心肌缺血再灌注CD40L表达的影响[J]. 北京中医药大学学报, 2010, 33(5):323-326. |
| [9] | Xi S G, Zhou G H, Zhang X X, et al. Protective effect of total aralosides of Aralia elata (Miq.) seem (TASAES) against diabetic cardiomyopathy in rats during the early stage, and possible mechanisms[J]. Exp Mol Med, 2009, 41(8):538-547. |
| [10] | Liu Y, Liu J F, Liu Z H, et al. The antitumor effects of araloside A extracted from the root bark of Aralia elataon human kidney cancer cell lines[J]. Afr J Pharm Pharmacol, 2011, 5(4):462-467. |
| [11] | 任丽, 路宝庭, 刘洋. 一种天然三萜皂苷的化学合成和抗肿瘤活性研究[J]. 中国药物化学杂志, 2011, 21(4):280-285. |
| [12] | Schmidt R R, Michael J. Facile synthesis of α- and β-O-glycosyl imidates; Preparation of glycosides and disaccharides[J]. Angew Chem Int Ed Engl, 1980, 19(9):731-732. |
| [13] | 郑利刚, 刘洋, 刘永祥, 等. 单糖活性给体合成方法的改进[J]. 沈阳药科大学学报, 2005, 22(5):352-354. |
 2016, Vol. 47
2016, Vol. 47


