多穗金粟兰Chloranthus multistachys Pei为金粟兰科(Chloranthaceae)金粟兰属Chloranthus Sw.植物,俗称白四块瓦、四大天王、四眼牛膝[1],常以根及根状茎入药,有祛湿散寒、理气活血、散瘀解毒等功效,有小毒,民间广泛用于治疗跌打骨折、腰腿痛、白带、疔肿、皮肤瘙痒等病症[2]。多穗金粟兰的化学成分研究较少,只有从多穗金粟兰中分离了少量萜类、香豆素、甾醇等化合物的报道[3-4]。而多穗金粟兰的同属植物如宽叶金粟兰、及己等的化学成分研究较多,尤其从宽叶金粟兰、及己中分离得到了一系列倍半萜二聚体类,该类化合物结构新颖且复杂,通常是以2分子倍半萜通过分子内的endo Diels-Alder环加成反应形成复杂的结构,另外结构中还往往具有α,β-不饱和γ-内酯或α,β不饱和酮的结构片段,部分化合物形成独特的大环结构,最大的为18元环[5]。图 1是一个乌药烷型倍半萜含18元大环内酯结构的二聚体,目前已从金粟兰属植物中分离得到了55个乌药烷型倍半萜二聚体类化
|
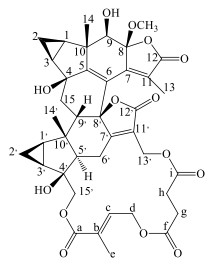
图 1 化合物 11 的化学结构 Fig.1 Structure of compound 11 |
合物,其中21个化合物含18元环结构。这类化合物大多数具有良好的生物活性[6],引起了国内外学者的广泛关注。
为了寻找多穗金粟兰中倍半萜二聚体类化合物,进一步开发利用该植物资源,本实验对多穗金粟兰根部氯仿和醋酸乙酯提取物进行了系统的分离研究,重点分离倍半萜二聚体类化合物。通过应用多种色谱技术从多穗金粟兰中分离得到12个乌药烷型倍半萜二聚体类化合物,分别鉴定为环银线草醇A-9-O-β-葡萄糖苷(9-O-β-glucopyranosylcycloshizukaol A,1)、环银线草醇A(cycloshizukaol A,2)、银线草醇B(shizukaol B,3)、银线草醇C(shizukaol C,4)、银线草醇D(shizukaol D,5)、银线草醇F(shizukaol F,6)、银线草醇G(shizukaol G,7)、多穗金粟兰醇B(chloramultiol B,8)、草珊瑚内酯B(sarcandrolide B,9)、sarglabolide I(10)、8-O-methyltianmushanol(11)、henriol B(12)。化合物1、7、9~12为首次从该植物中分离得到,化合物9和10为首次从金粟兰属植物中分离得到。
1 仪器与材料AB SCIEX TripleTOF 5600+高分辨飞行时间质谱联用仪(美国Sciex公司);Bruker AVANCE III HD 600 MHz核磁共振仪(瑞士Bruker公司);薄层色谱硅胶GF254和柱色谱用硅胶(100~200目,200~300目)均为青岛海洋化工厂产品;D101型大孔吸附树脂为天津海光化工有限公司生产;葡聚糖凝胶Sephadex LH-20为Amersham Pharmacia Biotech公司产品;Buchi R-205型旋转蒸发仪(瑞士Buchi公司);Waters半制备型液相色谱仪(美国Waters公司),制备型反相色谱柱为YMC-Pack ODS-A C18,(250 mm×20 mm,5 μm),分析型反相色谱柱为Kromasil 100A C18(150 mm×4.6 mm,5 μm);所用试剂均为分析纯或色谱纯。
药材于2014年11月采自江西井冈山市,由江西中医药大学赖学文教授鉴定为金粟兰科金粟兰属植物多穗金粟兰Chloranthus multistachys Pei,凭证标本保存于江西中医药大学标本馆(20141116)。
2 提取与分离干燥的多穗金粟兰根部10.5 kg,粉碎后用95%乙醇冷浸提取3次。提取液减压回收溶剂得到总浸膏312 g。总浸膏经硅胶减压柱分离,用石油醚、氯仿、醋酸乙酯、丙酮、甲醇依次洗脱,减压回收溶剂后得到石油醚部位(13 g)、氯仿部位(25 g)、醋酸乙酯部位(48 g)、丙酮部位(52 g)和甲醇部位(163 g)。
氯仿部位(25 g)经硅胶减压柱分离,以石油醚-醋酸乙酯(40:1→0:1)梯度洗脱,得到8个组分(LA~LH)。LD(5.0 g)经ODS分别用甲醇-水(30%、50%、70%、100%)洗脱,得到4个组分(LD1~LD4),其中LD2经制备液相(70%甲醇-水)分离,得到化合物2(89.1 mg)。LE(6.1 g)经硅胶色谱柱分离,以石油醚-丙酮(40:1→1:1)梯度洗脱,得到7个组分(LE1~LE7),其中E5经制备液相(67%甲醇-水)分离,得到化合物3(95.3 mg)、7(15.6 mg)。
醋酸乙酯部位(48 g)经硅胶减压柱分离,以石油醚-丙酮(10:1→0:1)梯度洗脱,得到5个组分(YA~YE)。YB(14.4 g)经硅胶色谱柱分离,以石油醚-丙酮(20:1→0:1)梯度洗脱,得到7个组分(YB1~YB7),其中YB4经制备液相(67%甲醇-水)分离,得到化合物4(45.0 mg)、5(35.5 mg)、6(12.9 mg);YC(14.4 g)经反相树脂分别用乙醇-水(30%、50%、70%、95%)洗脱,得到4个组分(YC1~YC4),YC1(5.1 g)经硅胶色谱柱分离,以石油醚-丙酮(10:1→0:1)梯度洗脱,得到5个组分(YC1-1~YC1-5),其中YC1-4经制备液相(60%甲醇-水)分离,得到化合物8(19.1 mg);YC2(8.0 g)经硅胶色谱柱分离,以石油醚-丙酮(20:1→0:1)梯度洗脱,得到6个组分(YC2-1~YC2-6),其中YC2-4经制备液相(50%甲醇-水)分离,得到化合物1(29.8 mg)、9(15.9 mg)、10(8.0 mg)、11(13.5 mg)、12(14.8 mg)。
3 结构鉴定化合物1:白色粉末,ESI-MS m/z: 733 [M+Na]+,C38H46O13。1H-NMR (600 MHz, CDCl3) δ: 2.00 (1H, m, H-1), 0.95 (1H, m, H-2α), 0.29 (1H, d, J=3.4 Hz, H-2β), 2.18 (1H, m, H-3), 6.97 (1H, s, H-6), 3.99 (1H, s, H-9), 1.53 (3H, s, H-13), 1.12 (3H, s, H-14), 3.31 (1H, d, J=13.5 Hz, H-15α), 2.20 (1H, d, J=13.5 Hz, H-15β), 3.69 (3H, s, 12-OMe), 1.88 (1H, m, H-1′), 0.90 (1H, m, H-2′α), 0.24 (1H, d, J=3.3 Hz, H-2′β), 2.23 (1H, m, H-3′), 6.97 (1H, s, H-6′), 3.62 (1H, s, H-9′), 1.52 (3H, s, H-13′), 1.10 (3H, s, H-14′), 3.28 (1H, d, J =13.2 Hz, H-15′α), 2.18 (1H, d, J=13.2 Hz, H-15′β), 3.66 (3H, s, 12′-OMe), 4.62 (1H, d, J=8.4 Hz, H-1″), 3.00 (1H, t, J=8.9 Hz, H-2″), 3.59 (1H, t, J=9.7 Hz, H-3″), 3.93 (1H, m, H-4″), 3.38 (1H, d, J=9.7 Hz, H-5″), 3.74 (1H, m, H-6″α), 3.83 (1H, m, H-6″β);13C-NMR (150 MHz, CDCl3) δ: 24.8 (C-1), 14.9 (C-2), 27.3 (C-3), 149.7 (C-4), 136.5 (C-5), 139.6 (C-6), 139.0 (C-7), 199.2 (C-8), 80.7 (C-9), 56.1 (C-10), 47.0 (C-11), 176.0 (C-12), 28.1 (C-13), 17.0 (C-14), 39.2 (C-15), 52.3 (12-OMe), 25.7 (C-1′), 14.4 (C-2′), 27.4 (C-3′), 148.7 (C-4′), 136.9 (C-5′), 139.3 (C-6′), 139.2 (C-7′), 198.7 (C-8′), 81.4 (C-9′), 55.8 (C-10′), 47.0 (C-11′), 176.1 (C-12′), 28.7 (C-13′), 16.4 (C-14′), 39.3 (C-15′), 52.4 (12′-OMe), 100.6 (C-1″), 72.5 (C-2″), 75.5 (C-3″), 67.7 (C-4″), 76.6 (C-5″), 60.8 (C-6″)。以上数据与文献报道基本一致[7],故鉴定化合物1为A-9-O-β-葡萄糖苷。
化合物2:无色透明块状结晶(甲醇),ESI-MS m/z: 571 [M+Na]+,C32H36O8。1H-NMR (600 MHz, CDCl3) δ: 1.84 (2H, m, H-1, 1′), 0.88 (2H, m, H-2α, 2α′), 0.23 (2H, m, H-2β, 2β′), 1.98 (2H, m, H-3, 3′), 7.18 (2H, s, H-6, 6′), 3.81 (2H, s, H-9, 9′), 1.52 (6H, s, H-13, 13′), 1.01 (6H, s, H-14, 14′), 2.97 (2H, d, J=13.2 Hz, H-15α, 15α′), 2.62 (2H, d, J=13.5 Hz, H-15β, 15β′), 3.65 (3H, s, H-OCH3);13C-NMR (150 MHz, CDCl3) δ: 24.7 (C-1, 1′), 14.0 (C-2, 2′), 27.9 (C-3, 3′), 147.4 (C-4, 4′), 135.8 (C-5, 5′), 138.4 (C-6, 6′), 137.3 (C-7, 7′), 199.0 (C-8, 8′), 81.0 (C-9, 9′), 58.4 (C-10, 10′), 47.8 (C-11, 11′), 175.5 (C-12, 12′), 28.5 (C-13, 13′), 16.0 (C-14, 14′), 38.3 (C-15, 15′), 52.1 (12, 12′-OMe)。以上数据与文献报道基本一致[8],故鉴定化合物2为环银线草醇A。
化合物3:白色粉末,ESI-MS m/z: 755 [M+Na]+,C40H44O13。1H-NMR (600 MHz, CDCl3) δ: 2.06 (1H, m, H-1), 1.02 (1H, m, H-2α), 0.33 (1H, m, H-2β), 1.89 (1H, m, H-3), 3.96 (1H, s, H-6), 3.88 (1H, s, H-9), 1.95 (3H, s, H-13), 1.04 (3H, s, H-14), 2.81 (1H, m, H-15α), 2.58 (1H, m, H-15β), 1.61 (1H, m, H-1′), 0.75 (1H, m, H-2′α), 1.35 (1H, m, H-2′β), 1.41 (1H, m, H-3′), 1.86 (1H, m, H-5′), 2.50 (1H, m, H-6′α), 2.70 (1H, m, H-6′β), 1.85 (1H, m, H-9′), 4.55 (1H, m, J =12.0 Hz, H-13′α), 5.10 (1H, d, J =12.0 Hz, H-13′β), 0.83 (3H, s, H-14′), 3.66 (1H, s, H-15′α), 4.62 (1H, d, J =12.0 Hz, H-15′β), 6.62 (1H, t, J =6.9 Hz, H-c), 4.64 (1H, m, H-dα), 5.08 (1H, m, H-dβ), 1.92 (3H, s, H-e), 2.48 (1H, m, H-gα), 2.90 (1H, m, H-gβ), 2.67 (1H, m, H-hα), 2.80 (1H, m, H-hβ), 3.71 (3H, s, 12-OMe);13C-NMR (150 MHz, CDCl3) δ: 26.0 (C-1), 16.0 (C-2), 24.8 (C-3), 142.4 (C-4), 132.1 (C-5), 41.0 (C-6), 131.5 (C-7), 200.8 (C-8), 79.9 (C-9), 50.9 (C-10), 147.3 (C-11), 170.2 (C-12), 20.1 (C-13), 15.3 (C-14), 25.4 (C-15), 25.6 (C-1′), 11.6 (C-2′), 27.7 (C-3′), 77.1 (C-4′), 61.3 (C-5′), 23.4 (C-6′), 174.7 (C-7′), 93.3 (C-8′), 55.5 (C-9′), 44.9 (C-10′), 123.3 (C-11′), 171.7 (C-12′), 54.3 (C-13′), 26.0 (C-14′), 72.2 (C-15′), 167.1 (C-a), 129.3 (C-b), 135.5 (C-c), 61.6 (C-d), 13.0 (C-e), 171.6 (C-f), 28.7 (C-g), 29.2 (C-h), 172.1 (C-i), 52.4 (12-OMe)。以上数据与文献报道基本一致[9],故鉴定化合物3为银线草醇B。
化合物4:黄色油状物,ESI-MS m/z: 657 [M+Na]+,C36H42O10。1H-NMR (600 MHz, CDCl3) δ: 2.06 (1H, m, H-1), 1.01 (1H, m, H-2α), 0.32 (1H, m, H-2β), 1.83 (1H, m, H-3), 3.90 (1H, d, J =3.5 Hz, H-6), 3.93 (1H, s, H-9), 1.90 (3H, s, H-13), 1.00 (3H, s, H-14), 2.81 (1H, m, H-15α), 2.57 (1H, m, H-15β), 1.60 (1H, m, H-1′), 0.71 (1H, m, H-2′α), 1.26 (1H, m, H-2′β), 1.51 (1H, m, H-3′), 1.91 (1H, m, H-5′), 2.27 (1H, m, H-6′α), 2.72 (1H, m, H-6′β), 2.05 (1H, m, H-9′), 3.31 (1H, d, J =13.6 Hz, H-13′α), 4.39 (1H, d, J =13.6 Hz, H-13′β), 0.86 (3H, s, H-14′), 3.85 (1H, d, J =11.5 Hz, H-15′α), 4.17 (1H, d, J =11.5 Hz, H-15′β), 6.87 (1H, qq, J=7.7, 3.3 Hz, H-c), 1.83 (1H, d, J =7.0 Hz, H-d), 1.84 (1H, brs, H-e), 3.76 (3H, s, 12-OMe);13C-NMR (150 MHz, CDCl3) δ: 26.2 (C-1), 16.0 (C-2), 24.8 (C-3), 142.5 (C-4), 132.2 (C-5), 41.0 (C-6), 131.3 (C-7), 200.3 (C-8), 79.9 (C-9), 51.0 (C-10), 147.0 (C-11), 171.2 (C-12), 20.3 (C-13), 15.5 (C-14), 25.3 (C-15), 25.4 (C-1′), 11.8 (C-2′), 28.3 (C-3′), 77.4 (C-4′), 60.0 (C-5′), 22.2 (C-6′), 168.5 (C-7′), 93.4 (C-8′), 54.7 (C-9′), 44.8 (C-10′), 127.4 (C-11′), 172.4 (C-12′), 55.0 (C-13′), 26.0 (C-14′), 71.0 (C-15′), 168.3 (C-a), 128.0 (C-b), 138.9 (C-c), 14.5 (C-d), 12.1 (C-e), 52.7 (12-OMe)。以上数据与文献报道基本一致[9],故鉴定化合物4为银线草醇C。
化合物5:黄色油状物,ESI-MS m/z: 589 [M+Na]+,C33H38O9。1H-NMR (600 MHz, CDCl3) δ: 2.06 (1H, m, H-1), 1.00 (1H, m, H-2α), 0.30 (1H, m, H-2β), 1.86 (1H, m, H-3), 3.91 (1H, d, J =3.8 Hz, H-6), 4.06 (1H, s, H-9), 1.90 (3H, s, H-13), 1.02 (3H, s, H-14), 2.76 (1H, m, H-15α), 2.60 (1H, m, H-15β), 1.45 (1H, m, H-1′), 0.77 (1H, m, H-2′α), 0.84 (1H, m, H-2′β), 1.10 (1H, m, H-3′α), 1.58 (1H, m, H-3′β), 1.81 (1H, dt, H-5′), 2.47 (1H, d, J =10.1 Hz, H-6′α), 2.47 (1H, d, J =10.1 Hz, H-6′β), 1.92 (1H, m, H-9′), 4.33 (1H, d, J =13.6 Hz, H-13′α), 4.39 (1H, d, J =13.6 Hz, H-13′β), 0.66 (3H, s, H-14′), 3.77 (1H, m, H-15′α), 3.98 (1H, m, H-15′β), 2.09 (3H, s, H-b), 3.79 (3H, s, 12-OMe);13C-NMR (150 MHz, CDCl3) δ: 25.7 (C-1), 15.9 (C-2), 24.8 (C-3), 142.4 (C-4), 131.7 (C-5), 40.6 (C-6), 131.5 (C-7), 200.7 (C-8), 79.9 (C-9), 51.1 (C-10), 147.0 (C-11), 171.1 (C-12), 20.5 (C-13), 15.3 (C-14), 25.6 (C-15), 24.3 (C-1′), 16.6 (C-2′), 21.8 (C-3′), 43.0 (C-4′), 59.2 (C-5′), 25.1 (C-6′), 168.7 (C-7′), 93.3 (C-8′), 54.5 (C-9′), 44.1 (C-10′), 126.7 (C-11′), 172.5 (C-12′), 54.9 (C-13′), 24.1 (C-14′), 66.2 (C-15′), 171.2 (C-a), 20.9 (C-b), 52.8 (12-OMe)。以上数据与文献报道基本一致[9],故鉴定化合物5为银线草醇D。
化合物6:黄色油状物,ESI-MS m/z: 755 [M+Na]+,C40H44O13。1H-NMR (600 MHz, CDCl3) δ: 2.09 (1H, m, H-1), 1.04 (1H, m, H-2α), 0.38 (1H, m, H-2β), 1.88 (1H, m, H-3), 3.96 (1H, d, J =3.2 Hz, H-6), 3.96 (1H, s, H-9), 1.94 (3H, s, H-13), 1.02 (3H, s, H-14), 2.82 (1H, m, H-15α), 2.58 (1H, m, H-15β), 1.65 (1H, m, H-1′), 0.73 (1H, m, H-2′α), 1.32 (1H, m, H-2′β), 1.40 (1H, m, H-3′), 1.96 (1H, m, H-5′), 2.66 (1H, m, H-6′α), 2.79 (1H, m, H-6′β), 1.95 (1H, m, H-9′), 4.72 (1H, d, J =12.7 Hz, H-13′α), 4.93 (1H, m, H-13′β), 0.81 (3H, s, H-14′), 3.52 (1H, d, J =11.8 Hz, H-15′α), 4.79 (1H, d, J =11.8 Hz, H-15′β), 5.98 (1H, m, H-b), 4.38 (1H, m, H-dα), 5.05 (1H, m, H-dβ), 2.15 (3H, s, H-e), 2.62 (1H, m, H-gα), 2.94 (1H, m, H-gβ), 2.77 (1H, m, H-hα), 2.87 (1H, m, H-hβ), 3.77 (3H, s, 12-OMe);13C-NMR (150 MHz, CDCl3) δ: 26.4 (C-1), 16.1 (C-2), 24.9 (C-3), 142.7 (C-4), 132.8 (C-5), 41.7 (C-6), 130.6 (C-7), 200.2 (C-8), 79.7 (C-9), 51.0 (C-10), 147.4 (C-11), 170.4 (C-12), 19.9 (C-13), 15.7 (C-14), 25.4 (C-15), 26.2 (C-1′), 11.7 (C-2′), 27.5 (C-3′), 77.8 (C-4′), 61.0 (C-5′), 24.7 (C-6′), 174.2 (C-7′), 93.5 (C-8′), 54.8 (C-9′), 44.8 (C-10′), 123.5 (C-11′), 171.5 (C-12′), 55.2 (C-13′), 25.8 (C-14′), 71.2 (C-15′), 166.2 (C-a), 112.8 (C-b), 154.0 (C-c), 65.7 (C-d), 15.6 (C-e), 171.8 (C-f), 29.1 (C-g), 29.0 (C-h), 172.0 (C-i), 52.5 (12-OMe)。以上数据与文献报道基本一致[10],故鉴定化合物6为银线草醇F。
化合物7:白色粉末,ESI-MS m/z: 771 [M+Na]+,C40H44O14。1H-NMR (600 MHz, CDCl3) δ: 2.08 (1H, m, H-1), 1.04 (1H, m, H-2α), 0.34 (1H, m, H-2β), 1.87 (1H, m, H-3), 3.97 (1H, d, H-6), 3.91 (1H, s, H-9), 1.89 (3H, s, H-13), 1.05 (3H, s, H-14), 2.78 (1H, m, H-15α), 2.61 (1H, m, H-15β), 1.62 (1H, m, H-1′), 0.76 (1H, m, H-2′α), 1.36 (1H, m, H-2′β), 1.42 (1H, m, H-3′), 1.87 (1H, m, H-5′), 2.62 (1H, m, H-6′α), 2.82 (1H, m, H-6′β), 1.83 (1H, m, H-9′), 4.45 (1H, d, J =11.9 Hz, H-13′α), 5.14 (1H, m, H-13′β), 0.83 (3H, s, H-14′), 3.62 (1H, d, J =11.9 Hz, H-15′α), 4.68 (1H, d, J =11.9 Hz, H-15′β), 6.76 (1H, m, H-b), 4.90 (1H, m, H-dα), 5.23 (1H, m, H-dβ), 1.95 (3H, s, H-e), 4.64 (1H, m, H-g), 2.92 (1H, m, H-hα), 2.99 (1H, m, H-hβ), 3.72 (3H, s, 12-OMe), 3.82 (1H, d, J =6.8 Hz, g-OH);13C-NMR (150 MHz, CDCl3) δ: 25.9 (C-1), 16.0 (C-2), 24.8 (C-3), 142.5 (C-4), 132.0 (C-5), 40.9 (C-6), 131.5 (C-7), 200.9 (C-8), 80.0 (C-9), 51.0 (C-10), 147.5 (C-11), 170.5 (C-12), 19.8 (C-13), 15.2 (C-14), 25.4 (C-15), 25.6 (C-1′), 11.7 (C-2′), 27.8 (C-3′), 77.3 (C-4′), 61.4 (C-5′), 24.1 (C-6′), 175.2 (C-7′), 93.4 (C-8′), 56.0 (C-9′), 45.0 (C-10′), 122.9 (C-11′), 171.8 (C-12′), 54.9 (C-13′), 26.1 (C-14′), 72.1 (C-15′), 167.3 (C-a), 129.0 (C-b), 135.8 (C-c), 62.2 (C-d), 13.1 (C-e), 173.4 (C-f), 67.2 (C-g), 38.9 (C-h), 170.7 (C-i), 52.8 (12-OMe)。以上数据与文献报道基本一致[10],故鉴定化合物7为银线草醇G。
化合物8:黄色颗粒,ESI-MS m/z: 577 [M+Na]+,C30H34O10。1H-NMR (600 MHz, Methanol-d4) δ: 1.83 (1H, m, H-1), 0.83 (1H, m, H-2α), 1.03 (1H, m, H-2β), 1.82 (1H, m, H-3), 3.70 (1H, s, H-9), 1.58 (3H, s, H-13), 0.86 (3H, s, H-14), 1.87 (1H, m, H-15α), 2.65 (1H, m, H-15β), 1.61 (1H, m, H-1′), 0.59 (1H, m, H-2′α), 1.17 (1H, m, H-2′β), 1.71 (1H, m, H-3′), 2.36 (1H, m, H-6′α), 2.89 (1H, m, H-6′β), 2.70 (1H, m, H-9′), 4.34 (1H, d, J =13.2 Hz, H-13′a), 4.28 (1H, m, H-13′b), 1.01 (3H, s, H-14′), 3.48 (2H, brs, H-15′);13C-NMR (150 MHz, Methanol-d4) δ: 28.2 (C-1), 8.1 (C-2), 30.1 (C-3), 78.5 (C-4), 163.2 (C-5), 123.3 (C-6), 153.9 (C-7), 103.6 (C-8), 77.3 (C-9), 49.6 (C-10), 123.5 (C-11), 171.9 (C-12), 9.3 (C-13), 12.7 (C-14), 40.4 (C-15), 26.3 (C-1′), 9.6 (C-2′), 28.7 (C-3′), 77.6 (C-4′), 51.1 (C-5′), 20.8 (C-6′), 169.7 (C-7′), 85.6 (C-8′), 51.0 (C-9′), 44.3 (C-10′), 127.0 (C-11′), 173.3 (C-12′), 53.2 (C-13′), 23.2 (C-14′), 66.9 (C-15′)。以上数据与文献报道基本一致[11],故鉴定化合物8为多穗金粟兰醇B。
化合物9:黄色油状物,ESI-MS m/z: 673 [M+Na]+,C36H42O11。1H-NMR (600 MHz, CDCl3) δ: 2.08 (1H, m, H-1), 0.34 (1H, m, H-2α), 0.99 (1H, m, H-2β), 1.87 (1H, m, H-3), 3.92 (1H, brs, H-6), 3.92 (1H, s, H-9), 1.91 (3H, s, H-13), 1.02 (3H, s, H-14), 2.58 (1H, m, H-15α), 2.83 (1H, m, H-15β), 1.65 (1H, m, H-1′), 0.73 (1H, m, H-2′α), 1.33 (1H, m, H-2′β), 1.45 (1H, m, H-3′), 1.90 (1H, m, H-5′), 2.41 (1H, m, H-6′α), 2.83 (1H, m, H-6′β), 1.95 (1H, m, H-9′), 4.34 (1H, d, J =15.3 Hz, H-13′α), 4.42 (1H, m, H-13′β), 0.88 (3H, s, H-14′), 3.82 (1H, d, J =11.6 Hz, H-15′α), 4.40 (1H, d, J =11.6 Hz, H-15′β), 6.79 (1H, m, H-c), 4.27 (1H, m, H-dα), 4.46 (1H, m, H-dβ), 1.89 (3H, s, H-e), 3.75 (3H, s, 12-OMe);13C-NMR (150 MHz, CDCl3) δ: 26.1 (C-1), 16.0 (C-2), 24.8 (C-3), 142.5 (C-4), 132.2 (C-5), 41.2 (C-6), 146.3 (C-7), 200.9 (C-8), 79.8 (C-9), 50.8 (C-10), 132.1 (C-11), 171.8 (C-12), 20.1 (C-13), 15.5 (C-14), 25.3 (C-15), 25.6 (C-1′), 11.6 (C-2′), 27.8 (C-3′), 77.4 (C-4′), 60.9 (C-5′), 23.1 (C-6′), 168.4 (C-7′), 93.1 (C-8′), 54.7 (C-9′), 44.8 (C-10′), 127.3 (C-11′), 172.3 (C-12′), 55.0 (C-13′), 26.1 (C-14′), 72.4 (C-15′), 167.7 (C-a), 127.4 (C-b), 141.9 (C-c), 59.6 (C-d), 12.6 (C-e), 53.2 (12-OMe)。以上数据与文献报道基本一致[12],故鉴定化合物9为草珊瑚内酯B。
化合物10:黄色油状物,ESI-MS m/z: 575 [M+Na]+,C31H36O9。1H-NMR (600 MHz, Methanol-d4) δ: 2.01 (1H, m, H-1), 0.30 (1H, m, H-2α), 1.00 (1H, m, H-2β), 1.73 (1H, m, H-3), 3.95 (1H, d, J =3.8 Hz, H-6), 4.05 (1H, s, H-9), 1.86 (3H, s, H-13), 1.05 (3H, s, H-14), 2.55 (1H, m, H-15α), 2.84 (1H, m, H-15β), 1.63 (1H, m, H-1′), 0.70 (1H, m, H-2′), 1.55 (1H, m, H-3′), 1.71 (1H, m, H-5′), 2.26 (1H, m, H-6′α), 2.79 (1H, m, H-6′β), 1.83 (1H, m, H-9′), 4.25 (1H, d, H-13′α), 4.27 (1H, d, H-13′β), 0.93 (3H, s, H-14′), 3.33 (1H, d, H-15′α), 3.38 (1H, d, J =10.9 Hz, H-15′β), 3.78 (3H, s, 12-OMe);13C-NMR (150 MHz, Methanol-d4) δ: 25.1 (C-1), 14.7 (C-2), 24.0 (C-3), 141.8 (C-4), 132.3 (C-5), 40.5 (C-6), 133.0 (C-7), 200.8 (C-8), 79.8 (C-9), 51.2 (C-10), 145.7 (C-11), 171.7 (C-12), 19.1 (C-13), 14.4 (C-14), 24.6 (C-15), 24.9 (C-1′), 11.3 (C-2′), 27.7 (C-3′), 78.1 (C-4′), 59.2 (C-5′), 21.6 (C-6′), 172.9 (C-7′), 93.3 (C-8′), 55.9 (C-9′), 44.4 (C-10′), 127.1 (C-11′), 170.2 (C-12′), 52.9 (C-13′), 25.8 (C-14′), 67.9 (C-15′), 51.7 (12-OMe)。以上数据与文献报道基本一致[13],故鉴定化合物10为sarglabolide I。
化合物11:白色颗粒,ESI-MS m/z: 771 [M+Na]+,C40H44O14。1H-NMR (600 MHz, DMSO-d4) δ: 1.68 (1H, m, H-1), 0.66 (1H, m, H-2α), 1.00 (1H, m, H-2β), 1.70 (1H, m, H-3), 3.71 (1H, s, H-9), 1.48 (3H, s, H-13), 0.72 (3H, s, H-14), 1.68 (1H, m, H-15α), 2.57 (1H, m, H-15β), 1.55 (1H, m, H-1′), 0.51 (1H, m, H-2′α), 1.08 (1H, m, H-2′β), 1.46 (1H, m, H-3′), 2.11 (1H, m, H-5′), 2.51 (1H, m, H-6′α), 2.77 (1H, m, H-6′β), 2.51 (1H, m, H-9′), 4.45 (1H, d, J =11.9 Hz, H-13′α), 4.86 (1H, d, J =11.9 Hz, H-13′β), 0.81 (3H, s, H-14′), 3.71 (1H, d, J =6.9 Hz, H-15′α), 4.60 (1H, d, J =6.9 Hz, H-15′β), 6.52 (1H, t, H-c), 1.77 (3H, s, H-d), 4.52 (1H, m, H-eα), 4.95 (1H, m, H-eβ), 2.41 (1H, m, H-gα), 2.54 (1H, m, H-gβ), 2.54 (1H, m, H-hα), 2.56 (1H, m, H-hβ), 3.18 (3H, s, 8-OMe);13C-NMR (150 MHz, DMSO-d4) δ: 28.8 (C-1), 9.0 (C-2), 31.0 (C-3), 78.1 (C-4), 164.0 (C-5), 122.6 (C-6), 153.8 (C-7), 104.1 (C-8), 76.3 (C-9), 49.1 (C-10), 122.0 (C-11), 172.7 (C-12), 10.5 (C-13), 14.7 (C-14), 40.5 (C-15), 26.8 (C-1′), 10.7 (C-2′), 29.2 (C-3′), 76.6 (C-4′), 54.9 (C-5′), 23.4 (C-6′), 176.7 (C-7′), 86.0 (C-8′), 49.8 (C-9′), 45.1 (C-10′), 122.0 (C-11′), 171.7 (C-12′), 54.4 (C-13′), 24.2 (C-14′), 73.5 (C-15′), 167.1 (C-a), 128.3 (C-b), 137.0 (C-c), 13.1 (C-d), 61.6 (C-e), 172.2 (C-f), 29.0 (C-g), 29.6 (C-h), 172.1 (C-i), 50.8 (8-OMe)。以上数据与文献报道基本一致[14],故鉴定化合物11为8-O-methyltianmushanol。
化合物12:黄色片状结晶(甲醇),ESI-MS m/z: 659 [M+Na]+,C35H40O11。1H-NMR (600 MHz, Methanol-d4) δ: 1.84 (1H, m, H-1), 0.82 (1H, m, H-2α), 1.05 (1H, m, H-2β), 1.83 (1H, m, H-3), 3.70 (1H, s, H-9), 1.57 (3H, s, H-13), 0.85 (3H, s, H-14), 1.85 (1H, m, H-15α), 2.70 (1H, m, H-15β), 1.68 (1H, m, H-1′), 0.62 (1H, m, H-2′α), 1.21 (1H, m, H-2′β), 1.74 (1H, m, H-3′), 2.35 (1H, m, H-5′), 2.45 (1H, m, H-6′α), 2.92 (1H, m, H-6′β), 2.71 (1H, m, H-9′), 4.33 (1H, m, H-13′), 1.03 (3H, s, H-14′), 4.01 (1H, d, J =11.0 Hz, H-15′α), 4.06 (1H, d, J =11.0 Hz, H-15′β), 6.97 (1H, m, H-c), 1.94 (3H, d, J =7.0 Hz, H-d), 1.84 (3H, s, H-e);13C-NMR (150 MHz, Methanol-d4) δ: 28.5 (C-1), 8.3 (C-2), 30.1 (C-3), 77.0 (C-4), 163.2 (C-5), 123.1 (C-6), 153.8 (C-7), 103.8 (C-8), 77.6 (C-9), 49.4 (C-10), 123.2 (C-11), 172.2 (C-12), 9.3 (C-13), 12.9 (C-14), 40.2 (C-15), 26.5 (C-1′), 9.6 (C-2′), 28.8 (C-3′), 76.9 (C-4′), 51.9 (C-5′), 20.8 (C-6′), 169.8 (C-7′), 85.7 (C-8′), 50.7 (C-9′), 44.4 (C-10′), 127.1 (C-11′), 173.4 (C-12′), 53.2 (C-13′), 23.2 (C-14′), 68.6 (C-15′), 168.1 (C-a), 128.6 (C-b), 137.3 (C-c), 11.0 (C-d), 13.1 (C-e)。以上数据与文献报道基本一致[15],故鉴定化合物12为henriol B。
4 讨论乌药烷型倍半萜二聚体类化合物结构新颖且复杂,大多数具有良好的生物活性,如抗真菌、对B细胞有毒性介导的免疫抑制及对延迟整流钾电流有选择性的抑制作用等[5]。另有研究显示,化合物2、3、6呈剂量依赖性抑制ICAM/LFA-1,TCAM/LFA-1的表达,有望成为有效的抗动脉粥样硬化和抗炎药物[16],化合物11有抑制络氨酸活性的作用[14]。
研究表明,α,β-不饱和γ-内酯和α,β不饱和酮均为细胞毒作用的有效基团[17]。本实验分离得到的12个乌药烷型倍半萜二聚体(其中化合物3、6、7、11含18元大环结构)均含有该基团,因此很有可能从中筛选出抗肿瘤的活性成分或先导化合物,为创新药物的开发奠定基础。本课题组将重点研究这些化合物的药理作用,包括18元大环结构在药理活性中的作用。
| [1] | 王兵娥, 王洪军. 白四块瓦形状与显微特征研究[J]. 中药材 , 2005, 28 (1) :17–18. |
| [2] | 中国科学院中国植物志编辑委员会. 中国植物志(第20卷)[M]. 北京: 科学出版社, 1982 . |
| [3] | 冉新辉.多穗金粟兰的化学成分研究[D].广州:广州中医药大学, 2010. http://cdmd.cnki.com.cn/article/cdmd-10572-2010126295.htm |
| [4] | 刘霞, 刘艳玲, 牛晓峰, 等. 多穗金粟兰化学成分研究[J]. 中药材 , 2012, 35 (8) :1254–1256. |
| [5] | 陈芳有, 张雪莲, 吴样明, 等. 金粟兰科植物萜类化学成分研究进展[J]. 江西中医药大学学报 , 2014, 26 (2) :89–94. |
| [6] | 乐贵洲, 杨立, 袁长春, 等. 乌药烷型倍半萜及其二聚体的全合成研究进展[J]. 有机化学 , 2012, 33 (1) :90–100. |
| [7] | Wang X C, Wang L L, Ouyang X W, et al. Sesquiterpenes and dimers thereof from Chloranthus fortune[J]. Helv Chim Acta , 2009, 92 (2) :313–320. DOI:10.1002/hlca.v92:2 |
| [8] | Kwon O E, Lee H S, Lee S W, et al. Dimeric sesquiterpenoids isolated from Chloranthus japonicus inhibited the expression of cell adhesion molecules[J]. J Ethnopharmacol , 2006, 104 (1) :270–277. |
| [9] | Kawabata J, Mizutani J. Dimeric sesquiterpenoid esters from Chloranthus serratus[J]. Phytochemistry , 1992, 31 (4) :1293–1296. DOI:10.1016/0031-9422(92)80281-I |
| [10] | Kawabata J, Fukushi E, Mizutani J. Sesquiterpene dimers from Chloranthus japonicus[J]. Phytochemistry , 1995, 39 (1) :121–125. DOI:10.1016/0031-9422(94)00865-Q |
| [11] | Ran X H, Teng F, Chen C X, et al. Chloramultiols A-F, Lindenane-type sesquiterpenoid dimers from Chloranthus multistachys[J]. J Nat Prod , 2010, 73 (5) :972–975. DOI:10.1021/np900764n |
| [12] | He X F, Yin S, Ji Y C, et al. Sesquiterpenes and dimeric sesquiterpenoids from Sarcandra glabra[J]. J Nat Prod , 2010, 73 (1) :45–50. DOI:10.1021/np9006469 |
| [13] | Wang P, Luo J, Zhang Y M, et al. Sesquiterpene dimers esterified with diverse small organic acids from the seeds of Sarcandra glabra[J]. Tetrahedron , 2015, 71 (33) :5362–5370. DOI:10.1016/j.tet.2015.05.112 |
| [14] | Wu B, Chen J, Qu H B, et al. Complex sesquiterpenoids with tyrosinase inhibitory activity from the leaves of Chloranthus tianmushanensis[J]. J Nat Prod , 2008, 71 (5) :877–880. DOI:10.1021/np070623r |
| [15] | Li C J, Zhang D M, Luo Y M, et al. Bis-sesquiterpenes and diterpenes from Chloranthus henryi[J]. Phytochemistry , 2008, 69 (16) :2867–2874. DOI:10.1016/j.phytochem.2008.08.022 |
| [16] | Kwon O, Lee H, Lee S. W, et al. Dimeric sesquiterpenoids isolated from Chloranthus japonicus inhibited the expression of cell adhesion molecules[J]. J Ethnopharmacol , 2006, 104 (1) :270–277. |
| [17] | 周伯庭. 我国金粟兰属植物化学成分和药理作用研究进展[J]. 中药材 , 2004, 27 (7) :539–542. |
 2016, Vol. 47
2016, Vol. 47


