甘草为豆科(Leguminosae)植物甘草Glycyrrhiza uralensis Fisch.、胀果甘草G. inflate Bat. 或光果甘草G. glabra L. 的干燥根及根茎,甘草主要分布于新疆和内蒙古[1];具有补脾益气、散热解毒、祛痰止咳、缓急止痛、调和诸药等众多功效[2]。现代药理学研究表明,甘草具有抗溃疡、抗炎[3]、抗菌、抗氧化、抗病毒、抗癌、抗抑郁、保肝[4]、祛痰和增强免疫力等多种药理活性[5]。甘草主要活性成分是三萜皂苷和黄酮类化合物[6,7],而国内外对于甘草地上部分的化学成分报道较少。本实验从甘草地上部分90%乙醇提取物中分离得到12个化合物,分别鉴定为(2S)-3′-(2-hydroxy-3-methylbut-3-enyl)-4′,5,7- trihydroxydihydroflavanone(1)、乔松素(pinocembrin,2)、sigmoidin B(3)、licoflavanone(4)、6-异戊烯基柚皮素(6-prenylnaringenin,5)、短叶松素(pinobanksin,6)、高良姜素(galangin,7)、染料木素(genistein,8)、红车轴草素(pratensein,9)、山柰酚-3-O-β-D-芸香糖苷(kaempferol-3-O-β-D- rutinoside,10)、芦丁(rutin,11)、α,α′-dihydro- 3,5,3′-trihydroxy-4′-methoxy-5′-isopentenyl-stilbene (12)。其中化合物1为新化合物,命名为羟甘草黄烷酮。化合物3、6、7和9是首次从甘草属植物中分离得到。
1 仪器与材料旋转蒸发仪(Eyela公司),低温循环水式多用真空泵(郑州长城科工贸有限公司),CD测定仪,JASCOJ-815旋光测定仪,液相色谱仪(Waters公司),TU-1810型紫外可见分光光度仪(北京普析通用仪器有限责任公司),中压液相色谱仪(Brucker 公司),400 MHz和600 MHz核磁共振仪(Varian公司)。Micromass Auto specultima ETOF型质谱仪;Aglient 1100 Series LC-MSD-Trap-SL型质谱仪。Sephadex LH-20(Pharmacia公司);柱色谱硅胶(100~200、200~300目)为青岛海洋化工厂产品,所有试剂均为分析纯,购自天津市福晨化学试剂厂。
实验材料于2014年5月采于新疆阿拉尔市塔里木大学一号教学楼前,经塔里木大学植物科学学院黄文娟副教授鉴定为甘草Glycyrrhiza uralensis Fisch.。标本(20140601)现存于塔里木大学生物研发中心320室。
2 提取与分离甘草地上部分13.5 kg,自然风干切成数小段,后用90%乙醇室温条件下浸泡7 d,减压浓缩回收溶剂后得浸膏552.7 g,乙醇浸膏用等量硅胶拌样后经硅胶柱色谱,三氯甲烷-甲醇溶剂系统(100∶1、10∶1、1∶1、1∶10、0∶1)梯度洗脱,按极性大小分为UA(36.8 g)、UB(74.4 g)、UC(134.6 g)、UD(77.2 g)和UE(83.1 g)。
组分UB经硅胶柱色谱,三氯甲烷-甲醇(50∶1、20∶1、10∶1、1∶1)梯度洗脱,按极性大小分为UBa(36.8 g)、UBb(74.4 g)、UBc(134.6 g)和UBd(77.2 g)。组分UBb经过制备液相分离得到化合物1(5.7 mg)、2(192.8 mg)、3(45.4 mg)和4(21.8 mg),组分UBc经硅胶柱(石油醚-丙酮2∶1)及制备液相(40%~70%甲醇)得化合物6(3.2 mg)、7(2.4 mg)和9(16.3 mg)。
组分UC经硅胶柱色谱,三氯甲烷-甲醇(10∶1→1∶1)梯度洗脱,按极性大小分为4个组分UCa(0.3 g)、UCb(3.7 g)、UCc(7.1 g)和UCd(56.5 g)。UCa经过凝聚柱(LH-20)分离得到化合物5(33.0 mg)。组分UCc再次过制备液相(40%~60%甲醇)分离得到化合物8(10.4 mg)和12(111.5 mg)。
组分UD经硅胶柱色谱,三氯甲烷-甲醇(1∶1→1∶10)梯度洗脱,按极性大小分为4个组分UDa(18.4 g)、UDb(25.7 g)、UDc(11.3 g)和UDd(8.6 g)。UDb经过凝聚柱(LH-20)分离得到化合物13(731.0 mg)。UDa再次过制备液相(40%~60%甲醇)分离得到化合物10(27.1 mg)和11(3.5 mg)。
3 结构鉴定化合物1:白色粉末,HR-TOF-MS m/z: 355.119 0 [M-H]−(C20H19O6,计算值355.118 2),提示其相对分子质量为356。CD谱 (MeOH): Δε283 nm−3.2,Δε333 nm +0.6。1H-NMR谱(表 1)中显示有3个双重峰δ 5.44 (1H,dd,J = 13.0,3.0 Hz),3.19 (1H,dd,J = 17.0,13.0 Hz) 和2.72 (1H,dd,J = 17.0,3.0 Hz),提示是1个二氢黄酮的特征吸收峰;另外,对芳香区光谱进行分析可以确定其芳香质子的取代形式,在A环上,有1个间位耦合的吸收峰δH 5.96 (2H,d,J = 2.0 Hz),提示是A环上1,2,3,5取代;而在B环上有1个ABX系统信号δ 6.87 (1H,d,J = 8.4 Hz),7.32 (1H,d,J = 2.0 Hz), 7.27 (1H,dd,J = 8.4,2.0 Hz),提示B环上1′,3′,4′取代。另外,1H-NMR谱还显示有2个亚甲基质子δH 2.90 (2H,m),1个次甲基质子δH 4.41 (1H,t,J = 6.0 Hz),和H-4″的2个单峰4.78 (1H,s),4.97 (1H,s)。这些信号与文献比对提示有2-hydroxy-3-methyl-but-3-enyl基[8]存在。加之在HMBC谱(图 1)中显示H-1″ [δH 2.90 (2H,d,J = 7.2 Hz)] 与 C-2′ (δC 131.0) 和C-4′ (δC 157.4) 相关,说明2-hydroxy-3- methyl-but-3-enyl基的取代位置是在C-3′。通过1H-NMR中的2号位质子的J = 13.0,3.0 Hz,说明2号位质子处于直立键;此外,在CD谱中283 nm为负效应,333 nm为正效应,确定C-2的绝对构型为S构型。综上所述,鉴定化合物1的结构为 (2S)-3′-(2-hydroxy-3-methylbut-3-eny)-4′,5,7- trihydroxy-dihydroflavanone,为1个新化合物,命名为羟甘草黄烷酮。
| 表 1 化合物1的1H-NMR和13C-NMR数据 (400/100 MHz,CD3COCD3) Table 1 1H-NMR and 13C-NMR data of compound 1 (400/100 MHz,CD3COCD3) |
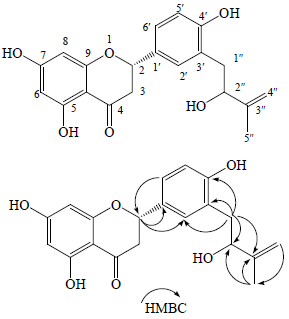 | 图 1 化合物1的结构和主要的HMBC相关 Fig.1 Structure and key HMBC correlations of compound 1 |
化合物2:淡黄色粉末。1H-NMR (400 MHz,CD3COCD3) δ: 12.17 (1H,brs,5-OH),9.59 (1H,brs,7-OH),7.57~7.58 (2H,m,H-2′,6′),7.38~7.48 (3H,m,H-3′~5′),6.01 (1H,d,J = 2.0 Hz,H-8),5.97 (1H,d,J = 2.0 Hz,H-6),5.58 (1H,dd,J = 13.0,3.0 Hz,H-2),3.18 (1H,dd,J = 13.0,3.0 Hz,H-3a),2.80 (1H,dd, J = 17.0,3.0 Hz,H-3b);13C-NMR (100 MHz,CD3COCD3) δ: 196.8 (C-4),166.4 (C-7),164.1 (C-5),163.3 (C-9),140.0 (C-1′), 129.5 (C-3′,5′),129.4 (C-4′),127.3 (C-2′,6′),103.2 (C-10),97.0 (C-8), 95.9 (C-6),79.9 (C-2),43.6 (C-3)。以上数据与文献报道一致[9],故鉴定化合物2为乔松素。
化合物3:淡黄色粉末。1H-NMR (400 MHz,CD3COCD3) δ: 12.17 (1H,brs,5-OH),6.91 (1H,s,H-2′),6.80 (1H,s,H-5′),5.95 (2H,s,H-6,8),5.35 (1H,dd,J = 13.0,3.0 Hz,H-2),5.34 (1H,t,J = 7.0 Hz,H-2″),3.35 (1H,d,J = 7.0 Hz,H-1″),3.11 (1H,dd,J = 13.0,3.0 Hz,H-3a),2.71 (1H,dd,J = 17.0,3.0 Hz,H-3b),1.71 (6H, s,H-4″,5″);13C-NMR (100 MHz,CD3COCD3) δ: 196.3 (C-4),166.4 (C-7),164.3 (C-5),163.4 (C-9),144.4 (C-5′),143.4 (C-4′),131.6 (C-3″),129.7 (C-1′),128.2 (C-3′),122.6 (C-2″),119.1 (C-2′),111.2 (C-6′), 102.3 (C-10),95.9 (C-6),95.0 (C-8),79.2 (C-2),42.6 (C-3),28.2 (C-1″),25.0 (C-4″),17.0 (C-5″)。以上数据与文献报道一致[10],故鉴定化合物3为sigmoidin B。
化合物4:淡黄色粉末。1H-NMR (400 MHz,CD3COCD3) δ: 12.19 (1H,brs,5-OH),7.28 (1H,d,J = 2.0 Hz,H-2′), 7.21 (1H,dd,J = 8.0,2.0 Hz,H-6′),6.89 (1H,d,J = 8.0 Hz, H-5′),5.95 (2H,s,H-6,8),5.42 (1H,dd,J = 13.0,3.0 Hz,H-2),5.36 (1H,t,J = 7.0 Hz,H-2″),3.35 (1H,d,J = 7.0 Hz,H-1″),3.18 (1H,dd,J = 13.0,3.0 Hz,H-3a),2.71 (1H,dd,J = 17.0,3.0 Hz, H-3b),1.71 (6H,s,H-4″,5″);13C-NMR (100 MHz,CD3COCD3) δ: 197.3 (C-4),167.3 (C-7),165.3 (C-5),164.4 (C-9),156.2 (C-4′), 132.7 (C-3″),130.8 (C-1′),129.1 (C-2″),129.0 (C-3′),126.3 (C-2′),123.5 (C-6′),115.7 (C-5′),103.2 (C-10),96.8 (C-6),95.8 (C-8),80.1 (C-2),43.5 (C-3),29.1 (C-1″),25.9 (C-4″),17.9 (C-5″)。以上数据与文献报道一致[9],故鉴定化合物4为licoflavanone。
化合物5:淡黄色固体。1H-NMR (400 MHz,CD3COCD3) δ: 12.19 (1H,brs,5-OH),9.57 (1H,brs,7-OH),7.57 (2H,d,J = 7.0 Hz,H-2′,6′),7.38~7.47 (3H,m,H-3′~5′),6.05 (1H,s,H-6),5.58 (1H,dd,J = 13.0,3.0 Hz,H-2), 5.40 (1H,t,J = 7.0 Hz,H-2″),3.22 (1H,d,J = 7.0 Hz,H-1″), 3.13 (1H,dd,J = 13.0,3.0 Hz,H-3a),2.84 (1H,dd,J = 17.0, 3.0 Hz,H-3b),1.62 (3H,s,H-5″),1.61 (3H,s,H-4″);13C-NMR (100 MHz,CD3COCD3) δ: 197.0 (C-4),164.8 (C-7),162.3 (C-5),161.8 (C-9),140.2 (C-1′),131.3 (C-3″),129.5 (C-2′,6′),129.4 (C-4′),127.3 (C-3′,5′),123.5 (C-2″),109.2 (C-8),103.2 (C-10),95.4 (C-6),79.9 (C-2),43.8 (C-3),25.9 (C-5″),21.7 (C-1″),17.8 (C-4″)。以上数据与文献报道一致[9],故鉴定化合物5为6-异戊烯基柚皮素。
化合物6:淡黄色针状结晶(甲醇)。1H-NMR (400 MHz,CD3COCD3) δ: 11.71 (1H,brs,5-OH),7.28 (1H,d,J = 2.0 Hz,H-2′),7.21 (1H,dd,J = 8.0,2.0 Hz,H-6′),6.89 (1H,d,J = 8.0 Hz,H-5′), 6.02 (1H,d,J = 2.0 Hz,H-8),5.99 (1H,d,J = 2.0 Hz,H-6), 5.19 (1H,d,J = 11.7 Hz,H-2),4.68 (1H,d,J = 11.7 Hz,H-3);13C-NMR (100 MHz,CD3COCD3) δ: 197.1 (C-4),166.9 (C-7),164.0 (C-5),163.2 (C-9),137.5 (C-1′),128.8 (C-4′),128.3 (C-3′,5′),128.0 (C-2′,6′),100.7 (C-10),96.3 (C-6),95.3 (C-8),83.6 (C-2), 72.3 (C-3)。以上数据与文献报道一致[11],故鉴定化合物6为短叶松素。
化合物7:淡黄色粉末。1H-NMR (400 MHz,CD3COCD3) δ: 12.10 (1H,brs,7-OH),8.26 (2H,d,J = 7.0 Hz,H-2′,6′), 7.51~7.59 (3H,m, H-3′~5′),6.58 (1H, s,H-6),6.30 (1H,s,H-8),2.84 (2H,s,5,3-OH);13C-NMR (100 MHz,CD3COCD3) δ: 176.1 (C-4),164.4 (C-7),161.4 (C-5),157.2 (C-9),145.3 (C-2),137.1 (C-3),131.1 (C-10),130.1 (C-4′),128.6 (C-2′,6′),127.7 (C-3′,5′),103.5 (C-1′),98.4 (C-6),93.8 (C-8)。以上数据与文献报道一致[12],故鉴定化合物7为高良姜素。
化合物8:无色针状结晶(甲醇)。1H-NMR (400 MHz,CD3COCD3) δ: 13.03 (1H,brs,5-OH),8.16 (1H,brs,7-OH),7.46 (2H,d,J = 8.3 Hz,H-2′,6′),7.45 (1H,s,H-2),6.91 (2H,d,J = 8.3 Hz,H-3′,5′), 6.43 (1H,s,H-8),6.29 (1H,s,H-6);13C-NMR (100 MHz,CD3COCD3) δ: 181.7 (C-4),165.0 (C-4′),163.9 (C-7),158.4 (C-9),154.3 (C-2), 124.0 (C-1′),123.1 (C-3),116.0 (C-3′),106.2 (C-10),99.9 (C-6),94.5 (C-8)。以上数据与文献报道一致[13],故鉴定化合物8为染料木素。
化合物9:无色针状结晶(甲醇)。1H-NMR (400 MHz,CD3COCD3) δ: 13.06 (1H,brs,5-OH),8.22 (1H,brs,7-OH),7.82 (1H,s,H-2),7.25 (1H,d,J = 1.5 Hz,H-2′),7.08 (1H,d,J = 8.0 Hz,H-6′),6.88 (1H,d,J = 8.0 Hz,H-5′),6.42 (1H,d,J = 1.5 Hz,H-6),6.29 (1H,d,J = 1.5 Hz,H-8),3.89 (1H,s,4′-OCH3);13C-NMR (100 MHz,CD3COCD3) δ: 181.6 (C-4),163.9 (C-5),159.0 (C-9),154.6 (C-2),148.0 (C-4′),147.7 (C-3′),124.1 (C-3),123.4 (C-1′),122.7 (C-6′),115.7 (C-5′),113.6 (C-2′),99.9 (C-6),94.5 (C-8),56.3 (C-OCH3)。以上数据与文献报道一致[14],故鉴定化合物9为红车轴草素。
化合物10:黄色粉末。1H-NMR (400 MHz,CD3OD) δ: 8.04 (2H,d,J = 8.5 Hz,H-2′,6′),6.87 (2H,d,J = 8.5 Hz,H-3′, 5′),6.39 (1H,d,J = 1.4 Hz,H-8),6.20 (1H,d,J = 1.4 Hz, H-6),5.11 (1H,d,J = 6.7 Hz,H-1″),4.50 (1H,d,J = 1.1 Hz, H-1′′′);13C-NMR (100 MHz,CD3OD) δ: 178.0 (C-4),164.6 (C-7),161.6 (C-5),160.1 (C-4′),158.0 (C-9),157.1 (C-2),134.1 (C-3),130.9 (C-2′,6′),121.3 (C-1′),114.7 (C-3′,5′),104.2 (C-1″),103.1 (C-10),101.0 (C-1′′′),98.5 (C-6),93.4 (C-8),76.7 (C-3″),75.8 (C-5″),74.2 (C-2″),72.4 (C-4′′′),70.8 (C-4″),70.6 (C-2′′′),70.0 (C-3′′′),68.3 (C-5′′′),67.1 (C-6″), 16.5 (C-6′′′)。以上数据与文献报道一致[15],故鉴定化合物10为山柰酚-3-O-β-D-芸香糖苷。
化合物11:黄色粉末。1H-NMR (400 MHz,CD3OD) δ: 7.65 (1H,d,J = 2.1 Hz,H-2′),6.08 (1H,dd,J = 8.4,2.1 Hz, H-6′),6.85 (1H,d,J = 8.4 Hz,H-5′),6.38 (1H,d,J = 2.1 Hz,H-8), 6.19 (1H,d,J = 2.1 Hz,H-6),5.09 (1H,d,J = 7.5 Hz,H-1″), 4.51 (1H,d,J = 0.9 Hz,H-1′′′);13C-NMR (100 MHz,CD3OD) δ: 177.9 (C-4),164.6 (C-7),161.5 (C-5),157.9 (C-9),157.0 (C-2),148.4 (C-4′), 144.4 (C-3′),134.2 (C-3),122.1 (C-6′),121.6 (C-1′),116.2 (C-5′),114.6 (C-2′), 104.2 (C-10),103.3 (C-1″),101.0 (C-1′′′),98.5 (C-6),93.4 (C-8),76.7 (C-3″), 75.8 (C-5″),74.3 (C-2″),72.5 (C-4′′′),70.8 (C-4″),70.7 (C-2′′′),69.9 (C-3′′′), 68.3 (C-5′′′),67.1 (C-6″),16.5 (C-6′′′)。以上数据与文献报道一致[16],故鉴定化合物11为芦丁。
化合物12:橘黄色油状物。1H-NMR (400 MHz,CD3COCD3) δ: 8.03 (2H,s,3′,5′-OH),7.78 (1H,s,3-OH),6.62 (1H,d,J = 2.0 Hz,H-2),6.51 (1H,d,J = 2.0 Hz,H-6),6.22 (2H,d,J = 2.1 Hz,H-2′,6′),6.19 (1H,t,J = 2.0 Hz,H-4′),5.26 (1H,t,J = 7.2 Hz,H-8),3.73 (3H,brs,4′-OCH3),3.28 (1H,d,J = 7.2 Hz,H-7),2.71 (4H,brs,H-α,β),1.72 (3H,s,H-11),1.71 (3H,s,H-10);13C-NMR (100 MHz,CD3COCD3) δ: 159.3 (C-3′), 150.6 (C-3),145.1 (C-1′),144.8 (C-4),138.7 (C-1),135.5 (C-5),132.1 (C-9), 124.4 (C-8),121.3 (C-6),115.0 (C-2),107.7 (C-2′),101.1 (C-4′),60.7 (-OCH3), 38.6 (C-β),37.9 (C-α),29.1 (C-7),25.9 (C-11),17.9 (C-10)。以上数据与文献报道一致[9],故鉴定化合物12为α,α′-dihydro-3,5,3′-trihydroxy- 4′-methoxy-5′-isopentenyl-stilbene。
| [1] | 刘洋洋,刘春生,曾斌芳,等.甘草种质资源研究进展[J].中草药, 2013, 44(24):3593-3598. |
| [2] | 中国药典[S].一部. 2010. |
| [3] | 张明发,沈雅琴.甘草及其活性成分抗炎与抗炎机制的研究进展[J].现代药物与临床, 2011, 26(4):261-268. |
| [4] | 张明发,沈雅琴.甘草及其有效成分抗脂肪肝和抗肥胖的研究进展[J].药物评价研究, 2015, 38(4):439-447. |
| [5] | Marjan N A, Hossein H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds[J]. Phytother Res, 2008, 22(6):709-724. |
| [6] | Wei S, Si L L, Ji S, et al. Uralsaponins M-Y, Antiviral triterpenoid saponins from the roots of Glycyrrhiza uralensis[J]. J Nat Prod, 2014, 77(7):1632-1643. |
| [7] | Zhou B, Wan C X. Phenolic constituents from the aerial parts of Glycyrrhiza inflata and their antibacterial activities[J]. J Asian Nat Prod Res, 2015, 17(3):256-261. |
| [8] | Long C, Derek T N, MinKyun N, et al. Isoprenylated flavonoids from the stem bark of Erythrina abyssinica[J]. J Nat Prod, 2007, 70(6):1039-1042. |
| [9] | Daniela M B, Concetta R, Giuseppe R. New dihydrostilbene derivatives from the leaves of Glycyrrhiza glabra and evaluation of their antioxidant activity[J]. J Nat Prod, 2003, 66(4):477-480. |
| [10] | Augustin E N, Kouam J, Voufo W T, et al. Further flavonoids from Erythrin species[J]. Phytochemistry, 1993, 32(4):1305-1311. |
| [11] | Han M S, Lee I K, Kim Y S, et al. Flavonoids from propolis inhibit DNA single strand breakage by the fenton reaction[J]. J Korean Soc Appl Biol Chem, 2010, 53(4):512-515. |
| [12] | Rajibul A L, Ismail S, Nayan R, et al. Antioxidant activity of Indian propolis and its chemical constituents[J]. Food Chem, 2010, 122(1):233-237. |
| [13] | 王青,苗文娟,向诚,等.乌拉尔甘草中黄酮类化学成分的研究[J].中草药, 2014, 45(1):31-36. |
| [14] | Ma X Q, Zheng C J, Zhang Y, et al. Antiosteoporotic flavonoids from Podocarpium podocarpum[J]. Phytochem Lett, 2013, 6(1):118-122. |
| [15] | 付伟,雷永芳,周道年,等.中日金星蕨黄酮类成分的研究[J].中国药学杂志, 2010, 45(3):166-168. |
| [16] | Wei X H, Yang S J, Liang N, et al. Chemical constituents of Caesalpinia decapetala(Roth) Alston[J]. Molecules, 2013, 18(1):1325-1336. |
 2016, Vol. 47
2016, Vol. 47



