红波罗花Incarvillea delavayi Bur. et Franch. 为紫葳科(Bignoniaceae)角蒿属Incarvillea Juss. 多年生草本植物,又名鸡肉参、土地黄、波罗花、红花角蒿,主要分布在四川(盐源、道孚)、云南西北部(大理、丽江、维西、中甸、德钦),生长于海拔2 900~3 600 m高山草甸和灌丛中。其根部作为鸡肉参入药,主治病后气血不足、头晕神疲、产后少乳[ 1,2 ]。红波罗花的化学成分以单萜生物碱、环己乙醇类及环烯醚萜类化合物为主。国外学者Nakamura等[ 3 ]从红波罗花的地上部分得到3个单萜生物碱delavayine A~C和1个环烯醚萜8-epideoxyloganic acid;卢涛[ 4 ]以采自云南洱源县焦石洞的红波罗花全草为研究对象,进行系统分离,获得了2个新的环烯醚萜[ 5,6 ]、2个新的酚类化合物以及1个结构新颖的天然产物杂合体incarviatone A[ 7 ]。陈玉琪[ 8,9 ]从云南格桑花卉种植基地的红波罗花中分离了1个新颖的环己乙醇二聚体incarviditone;卢龙海等[ 10 ]对红波罗花的醋酸乙酯部位进行了研究。为进一步研究红波罗花的化学成分,本实验对红波罗花全草的化学成分进行了研究,共分离了14个化合物,分别鉴定为5-羟乙基-6-羟基-3-甲基苯并呋喃(5- hydroxyethyl-6-hydroxyl-3-methyl benzofuran,1)、cleroindicin B(2)、3,4,5-三甲氧基苯甲酸乙酯(3,4,5-trimethoxy-benzoic acid ethyl ester,3)、3,4,5-三甲氧基苯甲酸甲酯(3,4,5-trimethoxyl benzoic acid methyl ester,4)、6-羟基苯并二氢呋喃(6-hydroxyl dihydrobenzofuran,5)、2-(4¢-乙氧基苯基)-乙醇 [2-(4¢-ethoxyphenyl)-ethanol,6]、tecomine(7)、(+)-epidihydrotecomanine(8)、5-hydroxy skytanthine(9)、δ-skytanthine(10)、isoincarvilline(11)、mairine B(12)、coelobillardierine(13)、3β-乙酰基齐墩果酸(3b-acetyl oleanolic acid,14)。结构见图 1。其中,化合物1为新化合物,命名为波罗花醇A;化合物3~6、9~11、13为首次从该植物中分离得到。
 | 图 1 化合物1~14的结构Fig. 1 Structures of compounds 1—14 |
Perkin-Elmer 341旋光仪(Perkin-Elmer公司);Varian CARY-50分光光度计(Varian 公司);Agilent 1260分析液相(Agilent 公司);岛津LC2010AHT分析液相(岛津公司);BUCHI C605中压液相系统(BUCHI公司)。Perkin-Elmer 577红外光谱仪(Perkin-Elmer公司)。Agilent LC/MSD Trap XCT及Agilent Q-TOF质谱仪(Agilent公司)。Bruker DRX-600型核磁共振仪(Bruker公司)。柱色谱填料:硅胶(200~300目,烟台汇友硅胶开发有限公司),Sephadex LH-20(Pharmacia公司),HSGF254色谱硅胶板(烟台芝罘黄务硅胶开发试验厂),反相ODS硅胶(YMC公司,50 μm)。提取用乙醇为工业试剂,液相色谱用试剂为色谱纯,其余试剂均为分析纯。
红波罗花全草于2010年9月采自云南省洱源县,经大理学院段宝忠副教授鉴定为紫葳科角蒿属植物红波罗花Incarvillea delavayi Bur. et Franch. 全草。标本(201009011)存放于第二军医大学药学院天然药物化学教研室。 2 提取与分离
红波罗花干燥全草18 kg,粉碎,以90%乙醇回流提取3次,每次 2 h,提取液减压浓缩成浸膏,加水稀释后用2%盐酸调pH值至2~3,滤过,分别得到滤液和滤渣,滤液加20% NaOH调pH值至11,用氯仿萃取,得到氯仿部位;水层和滤渣合并后,调pH值至7,用醋酸乙酯萃取,分别得到醋酸乙酯部位和水部位。氯仿部位经反复中性氧化铝柱色谱,石油醚-丙酮(100∶0→0∶100)梯度洗脱,Sephadex LH-20柱色谱,反相硅胶(Merck)以及半制备液相色谱分离,得到化合物7(9.6 mg)、8(120.6 mg)、9(61.9 mg)、10(20.6 mg)、11(530.0 mg)、12(115.0 mg)、13(5.6 mg);醋酸乙酯部位反复硅胶柱色谱,以石油醚-醋酸乙酯(100∶0→0∶100)梯度洗脱,获得6个馏份。馏份1经Sephadex LH-20柱色谱(CHCl3-MeOH 1︰1),反相硅胶(Merck)柱色谱(30%~100%甲醇水溶液梯度洗脱)以及半制备液相色谱(MeOH-H2O 8︰2)进行分离,得到化合物1(9.4 mg)、2(127.6 mg)、3(5.5 mg);馏份3经反相硅胶柱色谱(30%~100%甲醇水溶液梯度洗脱)以及半制备液相色谱(MeOH-H2O 65︰35)分离,得到化合物4(3.8 mg)、5(102.3 mg)、6(29.8 mg);通过类似方法,从馏份6中分离了化合物14(12.3 mg)。 3 结构鉴定
化合物1:黄色油状液体,ESI-MS给出准分子离子峰为m/z 215 [M+Na]+,高分辨HR-ESI-MS给出准分子离子峰m/z 215.068 5 [M+Na]+(计算值为215.068 4),相对分子质量为192,确定分子式为C11H12O3,不饱和度为6。UV谱在205、219 nm处显示最大吸收,IR谱显示该化合物含有羟基(3 383.32 cm-1)、苯环(1 614.13、1 565.91 cm-1)、甲基(2 956.34、1 432.85、1 388.50 cm-1)等基团。1H-NMR (400 MHz,C5D5N)(表 1)中给出1个单峰甲基质子信号δH 2.31 (3H,s)、2个裂分为三重峰的亚甲基质子信号δH 3.55 (2H,t,J = 6.8 Hz),4.33 (2H,t,J = 6.8 Hz)、1个单峰δH 6.52和2个裂分为二重峰δH7.14,7.34的烯氢质子信号。13C-NMR (100 MHz,C5D5N) 以及DEPT谱(表 1)中显示11个碳信号,包括1个甲基碳信号δC 14.1、2个亚甲基碳信号δC32.8,62.7(其中δC 62.7为连羟基的亚甲基碳信号)、3个次甲基碳信号δC102.5,109.7,113.1、5个季碳信号δC117.9,131.6,149.8,152.6,156.1,表明存在1个苯环及1个双键。通过HMQC谱归属了质子及与其直接相连的碳信号。在HMBC谱(图 2)中,甲基质子δH 2.31 (3H,s) 与δC 156.1 (C-2),102.5 (C-3) 显示相关信号,推测2位为甲基取代。亚甲基质子δH 3.55 (2H,t,J = 6.8 Hz) 与δC 131.6 (C-4),117.9 (C-5),152.6 (C-6),62.7 (C-11) 相关,以及含氧亚甲基质子δH4.33 (2H,t,J = 6.8 Hz) 与δC117.9 (C-5),32.8 (C-10) 相关,说明C-5被1个羟乙基取代,C-6位为1个羟基取代。根据以上信息,化合物1的结构确定为5-羟乙基-6-羟基-3-甲基苯并呋喃。经SciFinder检索,为1个新化合物,命名为波罗花醇A。
| 表 1 化合物1的1H-NMR 和13C-NMR 数据 Table 1 1H-NMR and 13C-NMR data of compound 1 |
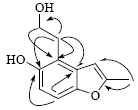 | 图 2 化合物1的重要HMBC相关图Fig. 2 Key HMBC correlations of compound 1 |
化合物2:淡黄色油状物,ESI-MS给出准分子离子峰为m/z 157 [M-H]-,相对分子质量为158,不饱和度为2,分子式为C8H14O3。1H-NMR (400 MHz,CDCl3) δ: 2.09~1.99 (2H,m,H-2a,6a),1.68 (2H,m,H-2b,6b),2.76~2.58 (2H,m,H-3a,5a),2.22~2.13 (2H,m,H-3b,5b),1.78~1.73 (2H,t,J = 5.8 Hz,H-7),3.88 (2H,t,J = 5.8 Hz,H-8);13C-NMR (100 MHz,CDCl3) δ: 70.6 (C-1),36.9 (C-2,6),37.1 (C-3,5),213.3 (C-4),41.8 (C-7),59.5 (C-8)。以上数据与文献报道一致[ 11 ],故鉴定化合物2为cleroindicin B。
化合物3:黄色油状体,ESI-MS给出准分子离子峰为m/z 263 [M+Na]+,503.1 [2M+Na]+,相对分子质量为240,分子式为C12H16O5。1H-NMR (400 MHz,CDCl3) δ: 7.28 (2H,s,H-2,6),4.35 (2H,q,J = 7.1 Hz,-OCH2CH3),1.38 (3H,t,J = 7.1 Hz,-OCH2CH3),3.89 (9H,s,3×-OCH3);13C-NMR (100 MHz,CDCl3) δ: 125.7 (C-1),106.9 (C-2,6),153.1 (C-3,5),142.3 (C-4),166.5 (C-7),61.4 (-OCH2CH3),14.6 (-OCH2CH3),56.4 (3,5-OCH3),61.1 (4-OCH3)。以上数据与文献报道一致[ 12 ],故鉴定化合物3为3,4,5-三甲氧基苯甲酸乙酯。
化合物4:黄色油状物,EI-MS显示准分子离子峰为m/z 249 [M+Na]+,475.1 [2M+Na]+,相对分子质量为226,分子式为C11H14O5。1H-NMR (500 MHz,CDCl3) δ: 7.28 (2H,s,H-2,6),3.88 (3H,s,-COOCH3),3.89 (9H,s,3×-OCH3);13C-NMR (125 MHz,CDCl3) δ: 125.4 (C-1),107.0 (C-2,6),153.1 (C-3,5),142.3.8 (C-4),167.0 (C-7),52.5 (-COOCH3),56.4 (3,5-OCH3),61.1 (4-OCH3)。以上数据与文献报道一致[ 13 ],故鉴定化合物4为3,4,5-三甲氧基苯甲酸甲酯。
化合物5:淡黄色固体,ESI-MS给出准分子离子峰为m/z 135 [M-H]-,相对分子质量为136,分子式为C8H8O2,不饱和度为5。1H-NMR (400 MHz,CD3OD) δ: 4.44 (2H,t,J = 8.6 Hz,H-2),3.10 (2H,t,J = 8.6 Hz,H-3),6.66 (1H,s,H-5),6.51 (1H,d,J = 8.4 Hz,H-7),6.50 (1H,d,J = 8.4 Hz,H-8);13C-NMR (100 MHz,CD3OD) δ: 72.2 (C-2),31.2 (C-3),129.3 (C-4),114.9 (C-5),152.3 (C-6),113.2 (C-7),110.0 (C-8),154.7 (C-9)。以上数据与文献报道一致[ 14 ],故鉴定化合物5为6-羟基苯并二氢呋喃。
化合物6:黄色油状物,EI-MS显示准分子离子峰为m/z 189 [M+Na]+,相对分子质量166,分子式为C10H14O2。1H-NMR (400 MHz,C5D5N) δ: 7.33 (2H,d,J = 8.6 Hz,H-2,6),6.99 (2H,d,J = 8.6 Hz,H-3,5),3.03 (2H,t,J = 7.0 Hz,H-7),3.92 (2H,t,J = 7.0 Hz,H-8),4.09 (2H,t,J = 7.0 Hz,-OCH2CH3),1.28 (3H,t,J = 7.0 Hz,-OCH2CH3);13C-NMR (100 MHz,C5D5N) δ: 132.6 (C-1),130.8 (C-2,6),115.1 (C-3,5),158.2 (C-4),39.9 (C-7),63. 8 (C-8),64.1 (-OCH2CH3),15.3 (-OCH2CH3)。对比文献报道中2-(4¢-乙氧基苯基)-乙醇的数据[ 15 ],鉴定化合物6为2-(4¢-乙氧基苯基)-乙醇。
化合物7:黄色油状物,Dragendorff试剂显色阳性,推测为生物碱类化合物。ESI-MS显示准分子离子峰为m/z 180 [M+H]+,202 [M+Na]+,相对分子质量为179,分子式为C11H17NO。1H-NMR (400 MHz,CDCl3) δ: 3.22 (1H,m,H-1a),1.72 (1H,t,J = 11.1 Hz,H-1b),2.98 (1H,m,H-3a),1.72 (1H,t,J = 11.1 Hz,H-3b),2.69 (1H,m,H-4),5.82 (1H,s,H-6),1.97~1.90 (1H,m,H-8),2.59~2.52 (1H,m,H-9),1.13 (3H,d,J = 6.6 Hz,H-10),1.16 (3H,d,J = 7.5 Hz,H-11),2.32 (1H,s,N-CH3);13C-NMR (100 MHz,CDCl3) δ: 63.3 (C-1),62.1 (C-3),35.2 (C-4),183.6 (C-5),124.4 (C-6),210.6 (C-7),45.2 (C-8),49.7 (C-9),15.0 (C-10),15.1 (C-11),45.6 (N-CH3)。以上数据与文献报道基本一致[ 16,17 ],故鉴定化合物7为tecomine。
化合物8:黄色油状物,Dragendorff试剂显色阳性,推测为生物碱类化合物。ESI-MS显示准分子离子峰为m/z 182 [M+H]+,204 [M+Na]+,相对分子质量为181,分子式为C11H19NO,不饱和度为3。1H-NMR (400 MHz,CDCl3) δ: 2.88 (1H,m,H-1a),2.27 (1H,t,J = 12.0 Hz,H-1b),2.70 (1H,m,H-3a),2.11 (1H,m,H-3b),1.44 (1H,m,H-4),1.63 (1H,m,H-5),2.20 (1H,m,H-6a),1.81 (1H,m,H-6b),2.45 (1H,m,H-8),1.57 (1H,m,H-9),1.01 (3H,d,J = 6.6 Hz,H-10),0.81 (3H,d,J = 6.4 Hz,H-11),2.10 (3H,s,N-CH3);13C-NMR (100 MHz,CDCl3) δ: 55.6 (C-1),62.9 (C-3),32.6 (C-4),43.7 (C-5),43.2 (C-6),221.1 (C-7),44.8 (C-8),39.2 (C-9),12.8 (C-10),17.7 (C-11),46.6 (N-CH3)。以上数据与文献报道一致[ 18 ],故鉴定化合物8为 (+)-epidihydrotecomanine。
化合物9:黄色油状物,Dragendorff试剂显色阳性,推测为生物碱类化合物,ESI-MS显示准分子离子峰为m/z 184 [M+H]+,206 [M+Na]+,相对分子质量为183,分子式为C11H21NO。1H-NMR (400 MHz,CDCl3) δ: 2.69 (1H,ddd,J = 11.6,5.9,1.8 Hz,H-1a),1.64~1.58 (1H,overlap,H-1b),2.58 (1H,ddd,J = 11.5,6.5,2.5 Hz,H-3a),1.95 (1H,m,H-3b),1.83 (1H,m,H-4),1.64~1.58 (1H,overlap,H-6a),1.37 (1H,m,H-6b),1.64~1.58 (1H,overlap,H-7a),1.42 (1H,m,H-7b),1.48 (1H,m,H-8),1.53 (1H,m,H-9),1.06 (3H,d,J = 7.1 Hz,H-10),0.81 (3H,d,J = 6.9 Hz,H-11),2.14 (3H,s,N-CH3);13C-NMR (100 MHz,CDCl3) δ: 61.7 (C-1),60.8 (C-3),39.5 (C-4),82.9 (C-5),30.4 (C-6),30.5 (C-7),37.1 (C-8),54.2 (C-9),24.0 (C-10),13.3 (C-11),45.8 (N-CH3)。以上数据与文献报道一致[ 19 ],故鉴定化合物9为5-hydroxy skytanthine。
化合物10:棕色油状物,Dragendorff试剂显色阳性,推测为生物碱类化合物。ESI-MS显示准分子离子峰为m/z 168 [M+H]+,相对分子质量为167,分子式为C11H21N。1H-NMR (400 MHz,CDCl3) δ: 2.85 (1H,d,J = 11.7 Hz,H-1a),2.08 (1H,dd,J = 11.4,3.6 Hz,H-1b),2.69 (1H,dd,J = 7.2,1.8 Hz,H-3a),2.12~2.07 (1H,m,H-3b),1.78~1.72 (1H,m,H-4),1.21~1.12 (1H,m,H-5),1.52~1.37 (2H,m,H-6),1.52~1.37 (2H,m,H-7),1.53 (1H,m,H-8),1.78~1.72 (1H,m,H-9),0.87 (3H,d,J = 6.2 Hz,H-10),1.01 (3H,d,J = 6.4 Hz,H-11),2.27 (3H,s,N- CH3);13C-NMR (100 MHz,CDCl3)δ: 55.3 (C-1),63.0 (C-3),32.4 (C-4),47.8 (C-5),27.1 (C-6),31.8 (C-7),33.2 (C-8),44.5 (C-9),19.0 (C-10),17.4 (C-11),46.4 (N-CH3)。以上数据与文献报道基本一致[ 20 ],故鉴定化合物10为δ-skytanthine。
化合物11:黄色油状物,Dragendorff试剂显色阳性,推测为生物碱类化合物。ESI-MS给出准分子离子峰为m/z 184 [M-H]-,206 [M+Na]+,相对分子质量为183,分子式为C11H21NO,不饱和度为2。1H-NMR (400 MHz,CDCl3) δ: 2.73 (1H,d,J = 12.0 Hz,H-1a),2.00 (1H,dd,J = 12.0,4.4 Hz,H-1b),2.59 (1H,dd,J = 11.1,3.5 Hz,H-3a),1.42 (1H,d,J = 11.1 Hz,H-3b),1.29 (1H,m,H-4),1.37 (1H,m,H-5),1.85 (1H,ddd,J = 14.5,6.6,1.7 Hz,H-6a),1.65 (1H,ddd,J = 14.3,7.8,2.3 Hz,H-6b),4.11 (1H,td,J = 6.2,2.3 Hz,H-7),1.94 (1H,m,H-8),1.70 (1H,t,J = 5.7 Hz,H-9),0.74 (3H,d,J = 6.3 Hz,H-10),0.91 (3H,d,J = 6.9 Hz,H-11),2.14 (3H,s,N-CH3);13C-NMR (100 MHz,CDCl3) δ: 55.6 (C-1),63.6 (C-3),34.7 (C-4),42.3 (C-5),39.6 (C-6),74.9 (C-7),38.9 (C-8),43.9 (C-9),17.9 (C-10),12.0 (C-11),47.0 (N-CH3)。以上数据与文献报道一致[ 21 ],故鉴定化合物11为isoinvarvilline。
化合物12:黄色油状物,Dragendorff试剂显色为阳性,推测为生物碱类化合物。ESI-MS给出准分子离子峰为m/z 184 [M-H]-,206 [M+Na]+,相对分子质量为183,不饱和度为2。1H-NMR (400 MHz,CDCl3) δ: 2.52 (1H,m,H-1a),1.63~1.54 (1H,m,H-1b),1.66 (1H,t,J = 11.6 Hz,H-3a),2.48~2.39 (1H,m,H-3b),2.11~1.97 (1H,m,H-4),1.87 (1H,m,H-5),1.39 (2H,m,H-6),1.15~1.02 (1H,m,H-7a),1.78 (1H,m,H-7b),1.78 (1H,m,H-8),2.11~1.97 (1H,m,H-9),0.79 (3H,d,J = 7.0 Hz,H-10),3.31 (2H,d,J = 7.2 Hz,H-11),2.16 (3H,s,N-CH3);13C-NMR (100 MHz,CDCl3) δ: 57.4 (C-1),58.0 (C-3),30.7 (C-4),41.2 (C-5),22.1 (C-6),26.0 (C-7),45.1 (C-8),40.8 (C-9),17.6 (C-10),66.3 (C-11),46.2 (N-CH3)。以上数据与文献报道一致[ 22 ],故鉴定化合物12为mairine B。
化合物13:无色油状物,Dragendorff试剂显色为阳性,推测为生物碱类化合物。ESI-MS给出准分子离子峰为m/z 172 [M+Na]+,相对分子质量为149,分子式为C9H11NO,不饱和度为5。1H-NMR (400 MHz,C5D5N) δ: 8.72 (1H,d,J = 8.8 Hz,H-2),7.16 (1H,d,J = 8.8 Hz,H-5),7.56 (1H,dd,J = 8.8,4.8 Hz,H-6),3.46 (1H,m,H-7),2.43 (1H,m,H-8a),2.03 (1H,m,H-8b),5.53 (1H,dd,J = 10.8,5.2 Hz,H-9),1.17 (3H,d,J = 7.2 Hz,H-10);13C-NMR (100 MHz,C5D5N) δ: 148.8 (C-2),144.4 (C-3),155.3 (C-4),120.5 (C-5),146.9 (C-6),36.1 (C-7),45.2 (C-8),74.2 (C-9),21.0 (C-10)。以上数据与文献报道一致[ 23 ],故鉴定化合物13为coelobillardierine。
化合物14:白色针晶(氯仿),mp 259~260 ℃;ESI-MS 给出准分子离子峰m/z 521 [M+Na]+,分子式为C32H50O4;Libermann-Burchard反应阳性,初步判定为三萜类化合物。1H-NMR (500 MHz,CDCl3) δ: 4.47 (1H,t,J = 7.8 Hz,H-3),5.24 (1H,s,H-12),0.82,0.83,0.87,0.90,0.91,1.10,1.22 (各3H,s,7×-CH3),2.02 (3H,s,-OCOCH3);13C-NMR (125 MHz,CDCl3)δ: 38.0 (C-1),32.5 (C-2),81.1 (C-3),38.2 (C-4),55.5 (C-5),18.4 (C-6),33.7 (C-7),39.5 (C-8),47.5 (C-9),37.2 (C-10),23.4 (C-11),122.7 (C-12),143.8 (C-13),41.7 (C-14),32.4 (C-15),22.8 (C-16),46.7 (C-17),40.8 (C-18),45.8 (C-19),30.7 (C-20),23.3 (C-21),27.6 (C-22),28.0 (C-23),16.9 (C-24),15.3 (C-25),17.3 (C-26),26.0 (C-27),183.1 (C-28),33.1 (C-29),23.6 (C-30),171.3,21.3 (-OCOCH3)。以上数据与文献报道一致[ 24 ],故鉴定化合物14为.3β-乙酰基齐墩果酸。 4 讨论
单萜生物碱和环己乙醇类化合物是角蒿属植物中2类较常见的化学成分,本实验从红波罗花中分离得到的化学成分也是以这2类成分为主,本研究也进一步丰富了红波罗花次生代谢产物结构的多样性。
| [1] | 国家中医药管理局《中华本草》编委会. 中华本草 [M]. 上海: 上海科学技术出版社, 1999. |
| [2] | 中国科学院中国植物志编辑委员会. 中国植物志 [M]. 北京: 科学出版社, 1990. |
| [3] | Nakamura M, Kido K, Kinjo J, et al. Antinociceptive substances from Incarvillea delavayi[J]. Phytochemistry, 2000, 53(2): 253-256. |
| [4] | 卢 涛. 红波罗花的化学成分与生物活性研究 [D]. 沈阳: 沈阳药科大学, 2008. |
| [5] | Lu T, Zhang W D, Pei Y H, et al. A new iridoid from Incarvillea delavayi[J]. Chin Chem Lett, 2007, 18(12): 1512-1514. |
| [6] | Lu T, Shen Y H, Lu M, et al. Three new compounds from Incarvillea delavayi[J]. Helv Chim Acta, 2009, 92(4): 768-773. |
| [7] | Shen Y H, Ding Y Q, Lu T, et al. Incarviatone A, a structurally unique natural product hybrid with a new carbon skeleton from Incarvillea delavayi, and its absolute configuration via calculated electronic circular dichroic spectra[J]. RSC Adv, 2012, 2(10): 4175-4180. |
| [8] | 陈玉琪. 红波罗花与丝毛瑞香的化学成分研究 [D]. 南京: 中国药科大学, 2008. |
| [9] | Chen Y Q, Shen Y H, Su Y Q, et al. Incarviditone: a novel cytotoxic benzofuranone dimer from Incarvillea delavayi Bureau et Franchet[J]. Chem Biodivers, 2009, 6(5): 779-783. |
| [10] | 卢龙海, 杨 明, 林 生, 等. 红波罗花醋酸乙酯部位化学成分研究[J]. 中国中药杂志, 2009, 34(14): 1799-1801. |
| [11] | Tian J, Zhao Q S, Zhang H J, et al. New cleroindicins from Clerodendrum indicum[J]. J Nat Prod, 1997, 60(8): 766-769. |
| [12] | Bao K, Fan A X, Dai Y, et al. Selective demethylation and debenzylation of aryl ethers by magnesium iodide under solvent-free conditions and its application to the total synthesis of natural products[J]. Org Biomol Chem, 2009, 7(24): 5084-5090. |
| [13] | Castillo-Avila G M, Garcia-Sosa K, Pena-Rodriguez L M. Antioxidants from the leaf extract of Byrsonima bucidaefolia[J]. Nat Prod Commun, 2009, 4(1): 83-86. |
| [14] | Graffe B, Sacquet M C, Maitte P. Synthese de composes comportant deux heterocycles oxygenes accoles au noyau[J]. J Heterocycl Chem, 1975, 12(2): 247-251. |
| [15] | Ramsden H E, Balint A E, Whitford W R, et al. Aryimagnesium chlorides: preparations and characterizations[J]. J Org Chem, 1957, 22(10): 1202-1206. |
| [16] | Costantino L, Raimondi L, Pirisino R, et al. Isolation and pharmacological activities of the Tecoma stans alkaloids[J]. Farmaco, 2003, 58(9): 781-785. |
| [17] | vidari G, Tripolini M, Novella P, et al. Desymmetrization of bicyclo [3.3.0] octane-3,7-dione by the Schmidt reaction: an easy synthesis of tecomanine[J]. Tetrahedron Asymmetry, 1997, 8(17): 2893-2903. |
| [18] | Cid M M, Pombo-Villar E. Enantioselective synthesis of 3-azabicyclo [4.3.0] nonane alkaloids[J]. Helv Chim Acta, 1993, 76(4): 1591-1607. |
| [19] | Lins A P, Felicio J D. Monoterpene alkaloids from Tecoma stans[J]. Phytochemistry, 1993, 34(3): 876-878. |
| [20] | Kaneda K, Honda T. Stereocontrolled synthesis of (+)-α-skytanthine by means of an intramolecular Pauson-Khand reaction[J]. Tetrahedron, 2008, 64(51): 11589-11593. |
| [21] | Su Y Q, Shen Y H, Lin S, et al. Two new alkaloids from Incarvillea mairei var. grandiflora[J]. Helv Chim Acta, 2009, 92(1): 165-170. |
| [22] | Xing A T, Tian J M, Liu C M, et al. Three new monoterpene alkaloids and a new caffeic acid ester from Incarvillea mairei var. multifoliolata[J]. Helv Chim Acta, 2010, 93(4): 718-723. |
| [23] | Lopez J L, Pusset J, San Feliciano A. Plantes de nouvelle-caledonie 115. alcaloides monoterpeniques de coelospermum billardieri[J]. J Nat Prod, 1988, 51(5): 829-835. |
| [24] | 王 韵, 司马硕丹, 李继霞, 等. 飞机草化学成分研究[J]. 中草药, 2012, 43(12): 2351-2355. |
 2015, Vol. 46
2015, Vol. 46


