南五味子Kadsura longipedunculata Finet et Gagnep为木兰科(Magnoliaceae)南五味子属Kadsura Kaempf. ex Juss. 植物,又名长梗南五味子、红木香、广福藤、紫金皮,产于江苏、安徽、浙江、江西等地[1]。其根部药用,味辛苦,性温,有活血消肿、祛风通络、理气止痛之功效;用于风湿性关节炎、跌打损伤、月经不调、中暑腹痛、溃疡病、胃肠炎等[2]。现代研究表明南五味子具有抗氧化、血小板活化因子拮抗活性[3]。
五味子是我国传统中药,来源于木兰科五味子属植物五味子Schisandra chinensis (Turcz.) Baill. 的干燥成熟果实,临床上五味子属植物常以果实入药,具有固涩收敛、益气生津、补肾宁心之功效[4];而南五味子属植物则常用根和藤茎药用,具有活血化瘀、行气止痛之功效,这主要是由于2个属药用植物化学成分差异造成的[5]。通过文献调研,发现五味子属和南五味子属植物的主要成分均是木脂素类和三萜类化合物。其中,螺苯骈呋喃型联苯环辛烯类木脂素是南五味子属的特征成分,其主要活性为抗凝血和抑制血小板聚集、钙离子拮抗等,为南五味子属植物作为活血化瘀药用的物质基础[3]。
为进一步寻找和阐明其活血消肿、祛风通络的活性成分,本实验对南五味子根的化学成分进行了深入系统的研究,从其95%乙醇提取物中分离得到16个化合物,其中包括10个木脂素类化合物、4个聚黄烷醇多酚类化合物、1个倍半萜苷和1个单萜苷类化合物,分别鉴定为pinobatol(1)、leptolepisol B(2)、(7S,8R)-4,7,9,9′-四羟基-3,3′-二甲氧基-8-O-4′-新木脂素(7S,8R-erythro-4,7,9,9′- tetrahydroxy-3,3′-dimethoxy-8-O-4′-neolignan,3)、2,3-二-(3-甲氧基-4,7-二羟基-苯基)-丁基-1,4-二醇 [2,3- bis-(α-hydroxy-4-hydroxy-3-methoxybenzyl)-butane- 1,4-diol,4]、(7′S,8R,8′S)-4,4′,9-三羟基-3,3′,5-三甲氧基-9′-O-β-D-吡喃木糖-2,7′-环木脂素 [(7′S,8R,8′S)- 4,4′,9-trihydroxy-3,3′,5-trimethoxy-9′-O-β-D-xylopyra-nosyl-2,7′-cyclo-lignan,5]、aviculin(6)、异落叶松脂素(ent-isolariciresinol,7)、lawsorosemarinol(8)、(+)-安五脂素 [(+)-anwulignan,9]、异落叶松脂素- 2α-O-β-D-木糖苷(isolariciresinol-2α-O-β-D-xyloside,10)、原花青定B3(procyanidin B3,11)、原飞燕草素B3(prodelphinidin B3,12)、(−)-棓儿茶素 [(−)- gallocatechin,13]、(+)-儿茶素 [(+)-catechin,14]、脱落酸-β-D-吡喃葡萄糖(abscisic acid-β-D- glucopyranosyl ester,15)、(−)-oleuropeic acid 8-O-β- D-glucopyranoside(16)。其中,化合物1~8、11~13、15、16为首次从该属植物中分得。
1 仪器与材料Agilent 1000 Series LC-MSD-Trap-SL型ESI-MS质谱仪(美国Agilent公司),Inova-500型核磁共振光谱仪(美国Varian公司),LabAlliance PREP100 高效液相色谱泵配备LabAlliance Model-210单波长紫外吸收检测器(美国科学系统),半制备色谱柱为5C18-PAQ(250 mm×10 mm,5 μm,日本Cosmosil公司),ODS柱色谱填料(日本YMC公司),葡聚糖凝胶Sephadex LH-20(瑞典GE Healthcare Bio-Science AB公司),柱色谱用硅胶和薄层色谱用硅胶(烟台市化学工业研究所生产)。所用试剂购自北京化工厂,级别为分析纯或色谱纯。
长梗南五味子根于2010年3月采自江西省九江县,经江西省科学院生物资源研究所九江县森林植物研究所谭策铭鉴定为南五味子Kadsura longipedunculata Finet et Gagnep的根。植物标本(样品编号ID-S-2591)保存于中国医学科学院药物研究所标本室。
2 提取与分离南五味子干燥根34 kg,用95%乙醇浸泡提取,乙醇加入量以刚好把药材浸没为准,每次浸泡24 h,共4次。合并上述滤液,50 ℃减压浓缩,得浸膏2 400 g。总浸膏2 400 g经硅胶柱色谱,依次用石油醚-丙酮(石油醚、50∶1、10∶1、5∶1、3∶1、1∶1、丙酮)、80%乙醇洗脱,得到15个部位。部位7和8经Sephadex LH-20凝胶柱色谱,洗脱剂为石油醚-氯仿-甲醇(5∶5∶1),再经过甲醇重结晶分离得到化合物9(109.3 g)。部位14和15的浸膏(1 024.5 g)分别加适量水分散混悬溶解,经醋酸乙酯萃取,分别得到部位14和15的醋酸乙酯部位及部位14和15的水部位。部位14的醋酸乙酯浸膏加适量水分散混悬溶解,滤去不溶物,滤液冻干(60 g),将冻干样品经ODS柱,甲醇-水(20%、40%、60%、80%甲醇)梯度洗脱,得到4个组分。其中组分14-1(1.5 g)经RP18 HPLC制备(17%甲醇)分离得到6个亚组分(A~F):亚组分14-1F(55 mg)经进一步RP18 HPLC制备(17%甲醇)分离得到化合物14(38 mg);亚组分14-1D(550 mg)经进一步RP18 HPLC制备(18%甲醇)分离得到化合物11(160 mg);亚组分14-1C(120 mg)经进一步RP18 HPLC制备(7%乙腈)分离得到化合物12(11 mg)和13(20 mg);组分14-2(3.4 g)经Sephadex LH-20凝胶柱色谱,洗脱剂为甲醇,得到7个亚组分(A~G):亚组分14-2D(160 mg)经甲醇重结晶得到化合物10(48 mg);亚组分14-2B(290 mg)经RP18 HPLC制备(30%甲醇)分离得到化合物15(14 mg);亚组分14-2C(1 g)经硅胶柱色谱,氯仿-甲醇(1∶0→1∶1)梯度洗脱,得到5个亚组分(C1~C5):亚组分14-2C-2(130 mg)经RP18 HPLC制备(28%甲醇)得到化合物8(41 mg);亚组分14-2C-3(130 mg)经RP18 HPLC制备(28%甲醇)得到化合物1(7 mg)和2(4 mg);亚组分14-2C-4(480 mg)经RP18 HPLC制备(27%甲醇)得到化合物5(10 mg)、6(4 mg)和16(18 mg)。亚组分14-2D(170 mg)经硅胶柱色谱,氯仿-甲醇(1∶0→1∶1)梯度洗脱,得到11个亚组分(D1~D11),亚组分14-2D-5(78 mg)经RP18 HPLC制备(24%甲醇)得到化合物3(8 mg)、4(7 mg)和7(7 mg)。
3 结构鉴定化合物1:白色粉末(甲醇)。ESI-MS m/z: 579 [M+Na]+。1H-NMR (500 MHz, CD3OD) δ: 7.06 (1H, d, J = 2.0 Hz, H-2), 7.05 (1H, dd, J = 9.5, 2.0 Hz, H-6′), 6.88 (1H, dd, J = 8.0, 2.0 Hz, H-6), 6.75 (1H, d, J = 8.0 Hz, H-5), 6.68 (1H, d, J = 8.0 Hz, H-5″), 6.66 (1H, d, J = 1.5 Hz, H-2″), 6.56 (1H, dd, J = 8.0, 1.5 Hz, H-6″), 6.23 (1H, d, J = 2.0 Hz, H-2′), 6.10 (1H, d, J = 9.5 Hz, H-5′), 5.04 (1H, d, J = 9.5 Hz, H-7), 4.74 (1H, d, J = 8.0 Hz, H-7′), 4.29 (1H, m, H-8′), 3.85 (3H, s, 3-OCH3), 3.78 (1H, dd, J = 12.0, 2.5 Hz, H-9′a), 3.72 (1H, dd, J = 12.0, 3.5 Hz, H-9′b), 3.68 (3H, s, 3″-OCH3), 3.63 (3H, s, 3′-OCH3), 3.48 (2H, t, J = 6.5 Hz, H-9″), 3.38 (2H, d, J = 6.0 Hz, H-9), 2.75 (1H, m, H-8), 2.52 (2H, t, J = 8.0 Hz, H-7″), 1.73 (2H, m, H-8″);13C-NMR (125 MHz, CD3OD) δ: 135.2 (C-1), 111.2 (C-2), 149.5 (C-3), 147.7 (C-4), 116.4 (C-5), 120.4 (C-6), 84.1 (C-7), 62.2 (C-8), 60.2 (C-9), 56.9 (C-1′), 113.9 (C-2′), 154.2 (C-3′), 184.0 (C-4′), 129.5 (C-5′), 151.3 (C-6′), 85.7 (C-7′), 80.9 (C-8′), 62.5 (C-9′), 137.7 (C-1″), 115.1 (C-2″), 153.8 (C-3″), 146.0 (C-4″), 117.1 (C-5″), 121.7 (C-6″), 32.9 (C-7″), 35.8 (C-8″), 62.5 (C-9″), 56.8 (3-OCH3), 55.6 (3′-OCH3), 56.3 (3″-OCH3)。以上数据与文献报道一致[6],故鉴定化合物1为pinobatol。
化合物2:白色粉末(甲醇)。ESI-MS m/z: 597 [M+Na]+。1H-NMR (500 MHz, CD3OD) δ: 6.95 (2H, brs, H-2′, 2), 6.80 (2H, brs, H-5, 6), 6.76 (1H, dd, J = 8.0, 1.5 Hz, H-6′), 6.72 (1H, d, J = 8.5 Hz, H-5″), 6.70 (1H, d, J = 1.5 Hz, H-2″), 6.67 (1H, d, J = 8.0 Hz, H-5′), 6.58 (1H, dd, J = 8.5, 1.5 Hz, H-6″), 4.76 (1H, d, J = 3.0 Hz, H-7), 4.75 (1H, d, J = 2.5 Hz, H-7′), 4.25 (1H, m, H-8), 4.20 (1H, m, H-8′), 3.78 (2H, dd, J = 11.5, 5.5 Hz, H-9′a, 9a), 3.74 (3H, s, 3′-OCH3), 3.68 (2H, overlap, H-9′b, 9b), 3.68 (6H, brs, 3, 3″- OCH3), 3.48 (2H, t, J = 6.5 Hz, H-9″), 2.52 (2H, t, J = 8.0 Hz, H-7″), 1.72 (2H, m, H-8″);13C-NMR (125 MHz, CD3OD) δ: 137.4 (C-1), 113.0 (C-2), 151.9 (C-3), 148.8 (C-4), 118.8 (C-5), 121.3 (C-6), 74.2 (C-7), 86.6 (C-8), 62.5 (C-9), 134.5 (C-1′), 112.1 (C-2′), 149.0 (C-3′), 147.3 (C-4′), 116.0 (C-5′), 121.2 (C-6′), 74.3 (C-7′), 86.8 (C-8′), 62.5 (C-9′), 138.4 (C-1″), 114.3 (C-2″), 152.1 (C-3″), 147.5 (C-4″), 119.8 (C-5″), 122.1 (C-6″), 33.0 (C-7″), 35.8 (C-8″), 62.5 (C-9″), 56.8 (3, 3′, 3″-OCH3)。以上数据与文献报道一致[7],故鉴定化合物2为leptolepisol B。
化合物3:白色粉末(甲醇)。ESI-MS m/z: 401 [M+Na]+。1H-NMR (500 MHz, CD3OD) δ: 6.60~6.94 (6H, H-2, 5, 6, 2′, 5′, 6′), 4.76 (1H, d, J = 6.0 Hz, H-7), 4.22 (1H, m, H-8), 3.79 (1H, dd, J = 12.0, 5.5 Hz, H-9a), 3.74 (3H, s, -OCH3), 3.73 (3H, s, -OCH3), 3.68 (1H, dd, J = 12.0, 3.5 Hz, H-9b), 3.49 (2H, t, J = 7.0 Hz, H-9′), 2.54 (2H, t, J = 7.0 Hz, H-7′), 1.73 (2H, m, H-8′);13C-NMR (125 MHz, CD3OD) δ: 134.5 (C-1), 112.1 (C-2), 149.0 (C-3), 147.3 (C-4), 115.9 (C-5), 121.3 (C-6), 74.4 (C-7), 87.0 (C-8), 138.4 (C-1′), 114.3 (C-2′), 152.2 (C-3′), 147.6 (C-4′), 120.0 (C-5′), 122.2 (C-6′), 33.0 (C-7′), 35.9 (C-8′), 62.5 (C-9, 9′), 56.8 (-OCH3), 56.6 (-OCH3)。以上数据与文献报道一致[8],故鉴定化合物3为 (7S,8R)-4,7,9,9′-四羟基-3,3′-二甲氧基-8-O-4′-新木脂素。
化合物4:白色粉末(甲醇)。EI-MS m/z: 394 [M]+。H-NMR (500 MHz, CD3OD) δ: 6.97 (2H, d, J = 2.0 Hz, H-2, 2′), 6.82 (2H, dd, J = 8.0, 2.0 Hz, H-6, 6′), 6.73 (2H, d, J = 8.0 Hz, H-5, 5′), 4.87 (2H, brd, J = 8.0 Hz, H-7, 7′), 3.82 (6H, s, -OCH3), 3.64 (2H, dd, J = 11.0, 3.0 Hz, H-9a, 9′a), 3.55 (2H, dd, J = 11.0, 5.0 Hz, H-9b, 9′b), 2.26 (2H, m, H-8, 8′);13C-NMR (125 MHz, CD3OD) δ: 135.3 (C-1, 1′), 111.5 (C-2, 2′), 149.4 (C-3, 3′), 147.7 (C-4, 4′), 116.3 (C-5, 5′), 120.8 (C-6, 6′), 84.7 (C-7, 7′), 55.7 (C-8, 8′), 62.1 (C-9, 9′), 56.7 (-OCH3)。以上数据与文献报道一致[9],故鉴定化合物4为2,3-二-(3-甲氧基-4,7-二羟基苯基)-丁基- 1,4-二醇。
化合物5:白色粉末(甲醇)。ESI-MS m/z: 545 [M+Na]+。1H-NMR (500 MHz, CD3OD) δ: 6.70 (1H, d, J = 2.0 Hz, H-2′), 6.59 (1H, d, J = 8.0 Hz, H-5′), 6.50 (1H, s, H-6), 6.44 (1H, dd, J = 8.0, 2.0 Hz, H-6′), 4.29 (1H, d, J = 6.5 Hz, H-7′), 4.15 (1H, d, J = 7.5 Hz, H-1″), 3.79 (3H, s, 3-OCH3), 3.77 (1H, overlap, H-5″a), 3.77 (1H, overlap, H-4″), 3.71 (3H, s, 3′-OCH3), 3.59 (1H, dd, J = 11.0, 4.0 Hz, H-9a), 3.48 (1H, dd, J = 11.0, 7.0 Hz, H-9b), 3.41 (1H, m, H-9′a), 3.36 (1H, dd, J = 10.0, 4.0 Hz, H-9′b), 3.28 (1H, overlap, H-5″b), 3.25 (3H, s, 5-OCH3), 3.16 (1H, t, J = 8.0 Hz, H-3″), 3.10 (1H, t, J = 11.0 Hz, H-2″), 2.66 (1H, dd, J = 15.0, 4.0 Hz, H-7a), 2.54 (1H, m, H-7b), 1.98 (1H, m, H-8′), 1.65 (1H, m, H-8);13C-NMR (CD3OD, 125 MHz) δ: 130.4 (C-1), 126.9 (C-2), 147.9 (C-3), 139.2 (C-4), 148.9 (C-5), 108.1 (C-6), 34.3 (C-7), 40.9 (C-8), 66.4 (C-9), 140.5 (C-1′), 113.9 (C-2′), 149.0 (C-3′), 145.6 (C-4′), 116.0 (C-5′), 122.0 (C-6′), 42.9 (C-7′), 47.2 (C-8′), 71.4 (C-9′), 105.8 (C-1″), 75.3 (C-2″), 78.3 (C-3″), 71.6 (C-4″), 67.3 (C-5″), 56.9 (3-OCH3), 60.3 (5-OCH3), 56.8 (3′-OCH3)。以上数据与文献报道一致[10],故鉴定化合物5为(7′S,8R,8′S)-4,4′,9-三羟基-3,3′,5-三甲氧基- 9′-O-β-D-吡喃木糖基基-2,7′-环木脂素。
化合物6:白色粉末(甲醇)。ESI-MS m/z: 529 [M+Na]+。1H-NMR (500 MHz, CD3OD) δ: 6.70 (1H, d, J = 8.0 Hz, H-5′), 6.61 (1H, s, H-6), 6.58 (1H, d, J = 2.0 Hz, H-2′), 6.54 (1H, dd, J = 8.0, 2.0 Hz, H-6′), 6.11 (1H, s, H-3), 4.45 (1H, brs, H-1″), 3.80~3.82 (2H, m, H-7′, 2″), 3.75~3.77 (1H, m, H-9′a), 3.75 (3H, s, -OCH3), 3.72 (3H, s, -OCH3), 3.68 (1H, dd, J = 10.5, 3.5 Hz, H-9a), 3.56~3.60 (2H, m, H-9, 3″), 3.46 (1H, m, H-5″), 3.29 (1H, t, J = 9.5 Hz, H-4″), 3.06 (1H, dd, J = 10.0, 4.0 Hz, H-9′b), 2.77 (2H, d, J = 7.5 Hz, H-7), 1.97 (1H, m, H-8), 1.81 (1H, m, H-8′), 1.13 (3H, d, J = 6.0 Hz, H-6″);13C-NMR (125 MHz, CD3OD) δ: 129.2 (C-1), 138.4 (C-2), 117.4 (C-3), 146.4 (C-4), 149.5 (C-5), 112.8 (C-6), 33.9 (C-7), 40.4 (C-8), 65.7 (C-9), 134.3 (C-1′), 113.9 (C-2′), 147.6 (C-3′), 145.6 (C-4′), 116.4 (C-5′), 123.5 (C-6′), 48.7 (C-7′), 45.8 (C-8′), 68.3 (C-9′), 102.6 (C-1″), 72.7 (C-2″), 72.9 (C-3″), 74.2 (C-4″), 70.5 (C-5″), 18.2 (C-6″), 56.7 (-OCH3)。以上数据与文献报道一致[11],故鉴定化合物6为aviculin。
化合物7:白色粉末(甲醇)。ESI-MS m/z: 383 [M+Na]+。1H-NMR (500 MHz, CD3OD) δ: 6.68 (1H, d, J = 8.0 Hz, H-5′), 6.62 (1H, d, J = 2.0 Hz, H-2′), 6.60 (1H, s, H-3), 6.56 (1H, dd, J = 8.0, 2.0 Hz, H-6′), 6.12 (1H, s, H-6), 3.75 (1H, d, J = 9.5 Hz, H-7′), 3.75 (3H, s, -OCH3), 3.72 (3H, s, -OCH3), 3.58~3.66 (3H, m, H-9a, 9b, 9′a), 3.34 (1H, dd, J = 11.5, 4.0 Hz, H-9′b), 2.72 (2H, d, J = 7.5 Hz, H-7), 1.94 (1H, m, H-8), 1.71 (1H, m, H-8′);13C-NMR (125 MHz, CD3OD) δ: 129.3 (C-1), 138.9 (C-2), 117.7 (C-3), 146.3 (C-4), 149.3 (C-5), 112.7 (C-6), 33.9 (C-7), 40.3 (C-8), 66.3 (C-9), 134.5 (C-1′), 114.1 (C-2′), 147.5 (C-3′), 145.6 (C-4′), 116.3 (C-5′), 123.5 (C-6′), 48.4 (C-7′/8′), 48.3 (C-7′/8′), 62.5 (C-9′), 56.7 (-OCH3), 56.6 (-OCH3)。以上数据与文献报道一致[12],故鉴定化合物7为异落叶松脂素。
化合物8:白色粉末(甲醇)。ESI-MS m/z: 279 [M+Na]+。1H-NMR (500 MHz, CD3OD) δ: 6.93 (1H, d, J = 7.5 Hz, H-5), 6.79 (1H, d, J = 1.5 Hz, H-2), 6.68 (1H, dd, J = 7.5, 1.5 Hz, H-6), 4.10 (1H, m, H-2′), 3.78 (3H, s, 3-OCH3), 3.69 (4H, m, H-1, 3′), 3.50 (2H, t, J = 6.5 Hz, H-9), 2.57 (2H, t, J = 8.0 Hz, H-7), 1.76 (2H, m, H-8);13C-NMR (125 MHz, CD3OD) δ: 138.6 (C-1), 114.4 (C-2), 152.3 (C-3), 147.1 (C-4), 119.8 (C-5), 122.2 (C-6), 33.0 (C-7), 35.9 (C-8), 62.5 (C-9), 62.3 (C-1′, 3′), 83.6 (C-2′), 56.7 (3-OCH3)。以上数据与文献报道一致[13],故鉴定化合物8为lawsorosemarinol。
化合物9:白色晶体(甲醇)。ESI-MS m/z: 351 [M+Na]+。1H-NMR (500 MHz, CD3COCD3) δ: 6.76 (1H, brs, H-2′), 6.74 (1H, d, J = 7.5 Hz, H-5′), 6.73 (1H, d, J = 8.0 Hz, H-5), 6.71 (1H, brs, H-2), 6.66 (1H, brd, J = 7.5 Hz, H-6′), 6.26 (1H, brd, J = 8.0 Hz, H-6), 5.93 (2H, d, J = 1.5 Hz, -OCH2O-), 3.81 (3H, s, -OCH3), 2.75 (2H, m, H-7a, 7′a), 2.28 (2H, m, H-7b, 7′b), 1.75 (2H, m, H-8, 8′), 0.83 (3H, d, J = 7.0 Hz, 8′-CH3), 0.82 (3H, d, J = 7.0 Hz, 8-CH3);13C-NMR (125 MHz, CD3COCD3) δ: 136.6 (C-1), 110.0 (C-2), 148.5 (C-3), 146.5 (C-4), 108.6 (C-5), 122.7 (C-6), 39.2 (C-7), 40.0 (C-8), 16.4 (C-9), 133.9 (C-1′), 113.2 (C-2′), 145.5 (C-3′), 148.1 (C-4′), 115.4 (C-5′), 122.3 (C-6′), 39.5 (C-7′), 40.1 (C-8′), 16.5 (C-9′), 56.2 (-OCH3), 101.6 (-OCH2O-)。以上数据与文献报道一致[14],故鉴定化合物9为 (+)-安五脂素。
化合物10:白色粉末(甲醇)。ESI-MS m/z: 515 [M+Na]+。1H-NMR (500 MHz, CD3OD) δ: 6.72 (1H, d, J = 2.0 Hz, H-2′), 6.68 (1H, d, J = 8.5 Hz, H-5′), 6.59 (1H, s, H-6), 6.57 (1H, dd, J = 8.5, 2.0 Hz, H-6′), 6.12 (1H, s, H-3), 3.99 (1H, d, J = 7.5 Hz, H-1″), 3.99 (1H, overlap, H-9a), 3.92 (1H, dd, J = 9.5, 2.5 Hz, H-9b), 3.76 (1H, overlap, H-7′), 3.75 (3H, s, 5-OCH3), 3.74 (3H, s, 3′-OCH3), 3.70 (1H, dd, J = 11.0, 3.5 Hz, H-9′a), 3.65 (1H, dd, J = 11.0, 6.5 Hz, H-9′b), 3.40 (1H, m, H-5″a), 3.23 (1H, t, J = 8.5 Hz, H-4″), 3.17 (1H, dd, J = 10.0, 3.5 Hz, H-5″b), 3.14 (1H, dd, J = 8.5, 7.0 Hz, H-3″), 3.06 (1H, dd, J = 11.5, 10.5 Hz, H-2″), 2.76 (2H, m, H-7), 2.01 (1H, m, H-8′), 1.79 (1H, m, H-8);13C-NMR (125 MHz, CD3OD) δ: 129.4 (C-1), 134.6 (C-2), 117.7 (C-3), 145.5 (C-4), 147.5 (C-5), 114.6 (C-6), 34.1 (C-7), 39.9 (C-8), 65.4 (C-9), 138.9 (C-1′), 112.7 (C-2′), 149.2 (C-3′), 146.2 (C-4′), 116.4 (C-5′), 123.4 (C-6′), 48.2 (C-7′), 46.2 (C-8′), 69.7 (C-9′), 106.1 (C-1″), 75.3 (C-2″), 78.2 (C-3″), 71.6 (C-4″), 67.2 (C-5″), 56.7 (3′-OCH3), 56.8 (5-OCH3)。以上数据与文献报道一致[15],故鉴定化合物10为异落叶松脂素-2α-O-β-D-木糖苷。
化合物11:白色粉末(甲醇)。ESI-MS m/z: 577 [M-H]−。该化合物存在2种构象不同的异构体,难以分离,结构见图 1。1H-NMR (500 MHz, CD3OD) δ: 6.89 (0.67H, d, J = 1.5 Hz), 6.74~6.79 (0.67H, m), 6.71 (0.33H, d, J = 8.0 Hz), 6.70 (0.33H, d, J = 8.0 Hz), 6.68 (0.67H, d, J = 1.5 Hz), 6.62 (0.67H, d, J = 8.0 Hz), 6.61 (0.67H, d, J = 8.0 Hz), 6.53 (0.67H, d, J = 1.5 Hz), 6.41 (0.67H, dd, J = 8.0, 1.5 Hz), 6.19 (0.67H, dd, J = 8.0, 1.5 Hz), 6.01 (0.67H, s), 5.88 (0.33H, s), 5.83 (0.67H, d, J = 2.0 Hz), 5.78 (0.33H, d, J = 2.0 Hz), 5.75 (0.33H, d, J = 2.0 Hz), 5.73 (0.67H, d, J = 2.0 Hz), 4.68 (0.33H, d, J = 7.0 Hz), 4.48 (0.67H, d, J = 7.5 Hz), 4.45 (0.67H, overlap, 4.35 (0.67H, d, J = 8.0 Hz), 4.30 (0.67H, d, J = 10.0 Hz), 4.28 (0.33H, d, J = 7.5 Hz), 4.20 (0.67H, d, J = 9.5 Hz), 4.01 (0.33H, m), 3.73 (0.67H, m), 2.77 (0.33H, dd, J = 16.5, 6.0 Hz), 2.71 (0.67H, dd, J = 16.5, 5.5 Hz), 2.53 (0.33H, dd, J = 16.5, 8.5 Hz), 2.43 (0.67H, dd, J = 16.5, 8.0 Hz);13C-NMR (125 MHz, CD3OD) δ: 158.9, 157.4, 156.2, 155.2, 146.4, 146.1, 145.9, 132.9, 132.2, 121.3, 120.9, 120.2, 116.7, 116.5, 116.4, 115.8, 115.5, 108.5, 107.5, 102.6, 97.8, 97.6, 97.2, 96.4, 84.3, 82.8, 74.0, 70.2, 69.2, 68.9, 38.9, 29.1。以上数据与文献报道一致[16],故鉴定化合物11为原花青定B3。
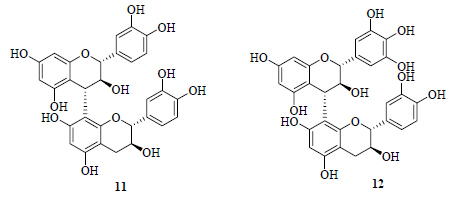 | 图 1 化合物11和12的结构Fig.1 Structures of compounds 11 and 12 |
化合物12:白色粉末(甲醇)。ESI-MS m/z: 593
[M-H]−。该化合物存在2种构象不同的异构体,难以分离,结构见图 1。1H-NMR (500 MHz, CD3OD) δ: 6.90 (0.5H, d, J = 2.0 Hz), 6.77 (0.5H, dd, J = 8.5, 2.0 Hz), 6.71 (0.5H, d, J = 8.0 Hz), 6.68 (0.5H, d, J = 2.0 Hz), 6.65 (0.5H, d, J = 8.0 Hz), 6.43 (0.5H, dd, J = 8.0, 1.5 Hz), 6.44 (1H, s), 6.07 (1H, s), 6.01 (0.5H, s), 5.88 (0.5H, s), 5.81 (0.5H, d, J = 2.5 Hz), 5.79 (0.5H, d, J = 2.0 Hz), 5.75 (0.5H, d, J = 2.0 Hz), 5.73 (0.5H, d, J = 2.5 Hz), 4.68 (0.5H, d, J = 7.0 Hz), 4.46 (1H, m), 4.37 (0.5H, d, J = 7.5 Hz), 4.32 (0.5H, t, J = 7.0 Hz), 4.31 (0.5H, overlap), 4.27 (0.5H, t, J = 8.0 Hz), 4.17 (0.5H, d, J = 10.0 Hz), 3.99 (0.5H, m), 3.75 (0.5H, m), 2.72 (1H, m), 2.53 (0.5H, dd, J = 16.0, 7.5 Hz), 2.42 (0.5H, dd, J = 16.5, 8.0 Hz);13C-NMR (125 MHz, CD3OD) δ: 159.0, 158.9, 157.5, 157.2, 156.2, 156.1, 155.8, 155.3, 155.1, 147.1, 146.7, 146.3, 145.8, 134.3, 133.0, 132.6, 131.8, 131.5, 129.2, 124.5, 121.3, 121.0, 116.7, 116.5, 116.2, 108.8, 108.4, 108.1, 107.7, 107.3, 105.0, 102.8, 100.6, 97.4, 96.0, 84.3, 84.2, 83.3, 83.1, 74.2, 74.0, 69.1, 68.8, 38.8, 29.2, 28.2。以上数据与文献报道一致[17],故鉴定化合物12为原飞燕草素B3。
化合物13:白色粉末(甲醇)。ESI-MS m/z: 305 [M-H]−。1H-NMR (500 MHz, CD3OD) δ: 6.34 (2H, s, H-2′, 6′), 5.86 (1H, d, J = 2.0 Hz, H-6), 5.80 (1H, d, J = 2.0 Hz, H-8), 4.47 (1H, d, J = 7.0 Hz, H-2), 3.90 (1H, m, H-3), 2.45 (1H, dd, J = 16.0, 7.0 Hz, H-4a), 2.75 (1H, dd, J = 16.0, 5.0 Hz, H-4b);13C-NMR (125 MHz, CD3OD) δ: 83.2 (C-2), 69.1 (C-3), 28.4 (C-4), 157.9 (C-5), 96.5 (C-6), 158.1 (C-7), 95.8 (C-8), 157.1 (C-9), 101.0 (C-10), 131.9 (C-1′), 107.5 (C-2′, 6′), 147.2 (C-3′, 5′), 134.3 (C-4′)。以上数据与文献报道一致[18],故鉴定化合物13为(−)-棓儿茶素。
化合物14:白色粉末(甲醇)。ESI-MS m/z: 289 [M-H]−。1H-NMR (500 MHz, CD3OD) δ: 6.78 (1H, d, J = 2.0 Hz, H-2′), 6.70 (1H, d, J = 8.5 Hz, H-5′), 6.66 (1H, dd, J = 8.5, 2.0 Hz, H-6′), 5.87 (1H, d, J = 2.0 Hz, H-6), 5.79 (1H, d, J = 2.0 Hz, H-8), 4.50 (1H, d, J = 7.5 Hz, H-2), 3.91 (1H, m, H-3), 2.45 (1H, dd, J = 16.0, 8.5 Hz, H-4a), 2.78 (1H, dd, J = 16.0, 5.5 Hz, H-4b);13C-NMR (125 MHz, CD3OD) δ: 82.8 (C-2), 68.8 (C-3), 28.5 (C-4), 157.5 (C-5), 96.3 (C-6), 157.8 (C-7), 95.5 (C-8), 156.9 (C-9), 100.8 (C-10), 132.2 (C-1′), 115.2 (C-2′), 146.2 (C-3′, 4′), 116.1 (C-5′), 120.0 (C-6′)。以上数据与文献报道一致[19],故鉴定化合物14为(+)-儿茶素。
化合物15:白色粉末(甲醇)。ESI-MS m/z: 449 [M+Na]+。1H-NMR (500 MHz, CD3OD) δ: 7.76 (1H, d, J = 16.0 Hz, H-4), 6.27 (1H, d, J = 16.0 Hz, H-5), 5.88 (1H, s, H-3′), 5.77 (1H, s, H-2), 5.44 (1H, d, J = 8.0 Hz, H-1″), 3.78 (1H, d, J = 12.5 Hz, H-6″a), 3.62 (1H, dd, J = 12.5, 5.0 Hz, H-6″b), 3.37 (1H, t, J = 8.0 Hz, H-4″), 3.25~3.32 (3H, m, H-2″, 3″, 5″), 2.49 (1H, d, J = 16.5 Hz, H-5′), 2.13 (1H, d, J = 16.5 Hz, H-5′), 2.02 (3H, s, H-6), 1.87 (3H, s, H-7′), 1.01 (3H, s, H-8′), 0.97 (3H, s, H-9′);13C-NMR (125 MHz, CD3OD) δ: 166.2 (C-1), 118.4 (C-2), 153.9 (C-3), 129.6 (C-4), 139.6 (C-5), 21.6 (C-6), 80.9 (C-1′), 166.6 (C-2′), 128.0 (C-3′), 201.2 (C-4′), 51.0 (C-5′), 43.2 (C-6′), 19.9 (C-7′), 23.9 (C-8′), 25.0 (C-9′), 95.7 (C-1″), 74.3 (C-2″), 78.4 (C-3″), 71.4 (C-4″), 79.1 (C-5″), 62.7 (C-6″)。以上数据与文献报道一致[20],故鉴定化合物15为脱落酸-β-D-吡喃葡萄糖苷。
化合物16:白色粉末(甲醇)。ESI-MS m/z: 369 [M+Na]+。1H-NMR (500 MHz, CD3OD) δ: 6.91 (1H, t, J = 2.5 Hz, H-2), 4.42 (1H, d, J = 7.5 Hz, H-1′), 3.76 (1H, dd, J = 11.5, 2.5 Hz, H-6′a), 3.60 (1H, dd, J = 11.5, 5.5 Hz, H-6′b), 3.30 (1H, t, J = 4.5 Hz, H-3′), 3.24 (1H, t, J = 7.5 Hz, H-4′), 3.17 (1H, m, H-2′), 3.08 (1H, t, J = 7.5 Hz, H-5′), 2.3~2.4 (2H, m, H-6a, 3a), 2.0~2.2 (3H, m, H-6b, 5a, 3b), 1.67 (1H, m, H-4), 1.20 (3H, s, H-9), 1.18 (3H, s, H-10), 1.14 (1H, m, H-5b);13C-NMR (125 MHz, CD3OD) δ: 132.2 (C-1), 141.1 (C-2), 28.9 (C-3), 44.8 (C-4), 24.8 (C-5), 26.7 (C-6), 171.4 (C-7), 80.8 (C-8), 23.6 (C-9), 25.1 (C-10), 98.8 (C-1′), 75.6 (C-2′), 78.6 (C-3′), 72.1 (C-4′), 77.8 (C-5′), 63.2 (C-6′)。以上数据与文献报道一致[21],故鉴定化合物16为(−)-oleuropeic acid 8- O-β-D-glucopyranoside。
4 结果与讨论本实验对南五味子根的化学成分进行了研究,其植物来源为南五味子,与《中国药典》2010年版中收载的药材南五味子不同,其植物来源为木兰科五味子属华中五味子Schisandra sphenanthera Rehd. et Wils的果实,二者同名,但一个为原植物名称,另一个为药材名称,十分容易混淆。
本实验从南五味子根中分离并鉴定了16个化合物,其中木脂素类化合物10个(1~10)、聚黄烷醇多酚类化合物4个(11~14)、倍半萜苷1个(15)和单萜苷1个(16)。
由于本实验分离部位极性较大,因此未发现联苯环辛烯类木脂素,主要分离得到芳基四氢萘类木脂素和简单木脂素。其中,化合物1为含有1个螺环的倍半木脂素类结构,该类结构极为少见,首次是从长白松Pinus sylvestris L. [6]中分离得到,目前为止在自然界发现该母核类结构化合物只有2个。(+)-安五脂素(化合物9)是南五味子中的主要成分,文献报道,其对人血清的乙肝表面抗原有较弱的抑制作用,在体外对H+, K+-ATP酶和P-388细胞有明显的抑制作用[22],并且对ADP诱导的血小板聚集有一定的抑制作用,可用作南五味子质量标准控制成分[23]。
首次从南五味子中发现聚黄烷醇多酚类化合物,聚黄烷醇多酚一般衍生于黄烷-3-醇,分子骨架为C6-C3-C6,常将该类化合物称原花色素。聚黄烷醇多酚根据其生源关系和骨架结构特点,分为三大类:单体黄烷醇、寡聚黄烷醇和红粉;再根据聚黄烷醇上下2个单元连接方式的不同,分为A型原花色素和B型原花色素。B型原花色素在天然产物中广泛分布,主要包括原花青定类化合物、原飞燕草色素类化合物、原花葵素类化合物等[24]。研究发现该类化合物具有多种药理活性,如调节血小板活性[25]、抗炎作用[16, 26]、抗癌活性[17]。
本实验首次从南五味子中发现的聚黄烷醇多酚类化合物,根据现有该类化合物活性研究的报道表明可能是南五味子具有活血化瘀、理气止痛等传统功效的又一主要物质基础,其药理活性值得进一步深入研究。
| [1] | 中国科学院中国植物志编辑委员会. 中国植物志[M]. 北京: 科学出版社, 1996. |
| [2] | 赵学敏. 本草纲目拾遗[M]. 北京: 人民卫生出版社, 1963. |
| [3] | 许利嘉, 刘海涛, 彭 勇, 等. 五味子科药用植物亲缘学初探[J]. 植物分类学报, 2008, 46(5): 692-723. |
| [4] | 史 琳, 王志成, 冯叙桥. 五味子化学成分及药理作用的研究进展[J]. 药物评价研究, 2011, 34(3): 208-212. |
| [5] | 陈道峰. 南五味子属药用植物的化学成分及其生物活性[J]. 中国天然药物, 2007, 5(1): 15-19. |
| [6] | Sinkkonen J, Liimatainen J, Karonen M, et al. A sesquineolignan with a spirodienone structure from Pinus sylvestris L.[J]. Angew Chem Int Ed, 2007, 46(22): 4148-4150. |
| [7] | Keiji M, Takashi M, Akira S. Structures of new lignans from Larix Leptolepis Gord.[J]. Tetrahedron Lett, 1979, 20(9): 799-802. |
| [8] | Park C H, Kim K H, Lee I K, et al. Phenolic constituents of Acorus gramineus[J]. Arch Pharmacal Res, 2011, 34(8): 1289-1296. |
| [9] | Pelter A, Ward R S, Waston D J, et al. Synthesis of 2,6-diary1-4,8-dihydroxy-3,7-dioxabicyclo[3.3.0] octanes[J]. J Chem Soc, Perkin Trans 1, 1982, 11(0): 175-182. |
| [10] | Pan J Y, Zhang S, Wu J, et al. Litseaglutinan A and lignans from Litsea glutinosa[J]. Helv Chim Acta, 2010, 93(5): 951-957. |
| [11] | Kim H, Woo E, Park H. A novel lignan and flavonoids from Polygonum Aviculare[J]. J Nat Prod, 1994, 57(5): 581-586. |
| [12] | Urones J G, Teresa J, Marcos I, et al. Ent-isolariciresinol in Reseda Suffruticosa[J]. Phytochemistry, 1987, 26(5): 1540-1541. |
| [13] | Uddin N, Siddiqui B S, Begum S, et al. Bioassay-guided isolation of urease and α-chymotrypsin inhibitory constituents from the stems of Lawsonia alba Lam. (Henna)[J]. Fitoterapia, 2013, 84(1): 202-207. |
| [14] | Jiang S J, Wang Y H, Chen D F. Sphenanlignan, a new lignan from the seeds of Schisandra sphenanthera[J]. Chin J Nat Med, 2005, 3(2): 78-82. |
| [15] | Xu J, Li F, Feng Z, et al. A new sesquiterpenoid from Mallotus apelta[J]. Chem Nat Compd, 2011, 47(2): 218-219. |
| [16] | Yukiko O, Yoshihiro M, Mitsuru H, et al. Synthesis of procyanidin B3 and its anti-inflammatory activity. The effect of 4-alkoxy group of catechin electrophile in the Yb (OTf)3-catalyzed condensation with catechin nucleophile[J]. J Org Chem, 2010, 75(14): 4884-4886. |
| [17] | Wataru F, Kazuya T, Koichiro K, et al. Syntheses of prodelphinidin B3 and C2, and their antitumor activities through cell cycle arrest and caspase-3 activation[J]. Tetrahedron, 2013, 69(17): 3543-3550. |
| [18] | Davis A L, Cai Y, Davies A P, et al. 1H and 13C NMR Assignments of some green tea polyphenols[J]. Magn Reson Chem, 1996, 34(11): 887-890. |
| [19] | Shen C C, Chang Y S, Hott L K. Nuclear magnetic resonance studies of 5, 7-dihydroxyflavonoids[J]. Phytochemistry, 1993, 34(3): 843-845. |
| [20] | Hisashi K N, Yukitoshi T, Toshihumi M, et al. Isolation and identification of an allelopathic substance from peel of Citrus junos[J]. Phytochemistry, 2002, 61(7): 849-853. |
| [21] | Tsutomu N, Naoki I, Yuka I, et al. A monoterpene glucoside and three megastigmane glycosides from Juniperus communis var. depressa[J]. Chem Pharm Bull, 2005, 53(7): 783-787. |
| [22] | 刘嘉森, 黄梅芬. (+)-安五脂素的分离与结构[J]. 有机化学, 1988, 8(3): 227-228. |
| [23] | 蒋仕丽, 章蕴毅, 陈道峰. 异型南五味子丁素、五味子酚和 (+)-安五脂素对血小板聚集的影响[J]. 复旦学报: 医学版, 2005, 32(4): 467-478. |
| [24] | 张东明. 酚酸化学[M]. 北京: 化学工业出版社, 2008. |
| [25] | Pütter M, Grotemeyer K H M, Würthwein G, et al. Inhibition of smoking-induced platelet aggregation by aspirin and pycnogenol[J]. Thromb Res, 1999, 95(4): 155-161. |
| [26] | Lei Y F, Ren X H, Chen J G, et al. Protective effects of grape seed-derived procyanidin extract against carrageenan-induced abacterial prostatitis in rats[J]. J Functional Foods, 2014, 7(1): 416-424. |
 2015, Vol. 46
2015, Vol. 46


