牡荆叶为马鞭草科(Verbenaceae)牡荆属Vitex L. 植物牡荆Vitex negundo L. var. cannabifolia (Sieb. et Zucc.) Hand. -Mazz. 的叶,在全国各地均有分布。牡荆的新鲜叶具有祛痰、止咳、平喘的功效,主要用于咳嗽痰多,鲜叶提取的挥发油可用于治疗慢性支气管炎[1]。牡荆的干燥叶具有解表化湿、祛痰平喘、解毒等功效,主要用于伤风感冒、咳嗽哮喘、胃痛、腹痛、暑湿泻痢、脚气肿胀、风疹瘙痒、脚癣、乳痈肿痛、蛇虫咬伤等[2]。文献报道,牡荆主要含有二萜[3, 4]、黄酮[3, 4, 5, 6]、环烯醚萜苷[3, 5]、木脂素[5]、酚苷[4, 5]及挥发性成分。目前,对牡荆叶的化学和药理活性研究多集中于其挥发油部分,而对其非挥发性成分研究报道较少。本课题组前期对牡荆干燥叶95%乙醇提取物的醋酸乙酯部位进行了系统的化学研究[7]。本实验从牡荆叶醋酸乙酯部位中分离得到7个黄酮苷类化合物,分别鉴定为木犀草素-4′-O- (6″-O-对羟基苯甲酰基)-β-D-葡萄糖苷 [luteolin-4′- O-(6″-O-p-hydroxybenzoyl)-β-D-glucoside,1]、木犀草素-7-O-(6″-O-对羟基苯甲酰基)-β-D-葡萄糖苷 [luteolin-7-O-(6″-O-p-hydroxybenzoyl)-β-D-glucoside,2]、木犀草素-6-C-(6″-O-反式-咖啡酰基)-β-D-葡萄糖苷 [luteolin-6-C-(6″-O-trans-caffeoyl)-β-D-glucoside,3]、木犀草素-6-C-(2″-O-反式-咖啡酰基)-β-D-葡萄糖苷 [luteolin-6-C-(2″-O-trans-caffeoyl)-β-D-glucoside,4]、perfoliatumin A(5)、异牡荆素(isovitexin,6)和木犀草素-7-O-β-D-葡萄糖苷(luteolin-7-O-β-D- glucoside,7)。化合物1为新化合物,化合物5为首次从牡荆属植物中分离得到,化合物2~4、7为首次从该植物中分离得到。
1 仪器与材料高效液相-离子阱-飞行时间质谱仪(日本岛津公司);Varian 500核磁共振仪(美国Varian公司);Sephadex LH-20填料(Amersham Biosciences,瑞典);ODS柱色谱填料(40~63 μm,德国Merck);硅胶GF254薄层预制板(烟台化学工业研究所);柱色谱用硅胶(200~300目)为青岛海洋化工厂生产,其他试剂均为分析纯。
牡荆叶于2012年9月采自河南省信阳市,由北京大学药学院屠鹏飞教授鉴定为牡荆Vitex negundo L. var. cannabifolia (Sieb. et Zucc.) Hand. -Mazz. 的叶。植物标本(编号JLI-VNC-201209)存放于北京中医药大学中药现代研究中心。
2 提取与分离牡荆叶(10.0 kg)用95%乙醇回流提取(3×100 L,每次3 h),提取液减压回收溶剂,得到总浸膏(3.6 kg),加适量水混悬,依次用石油醚、醋酸乙酯、正丁醇萃取,得到石油醚部位(310 g)、醋酸乙酯部位(350 g)、正丁醇部位(1 300 g)。
醋酸乙酯部位(290 g)经硅胶柱色谱分离,二氯甲烷-甲醇(20∶1→0∶1)梯度洗脱得到12个流分(Fr. 1~12)。Fr. 8经硅胶柱色谱(200~300目)分离,得9个流分(Fr. 8A~8I)。Fr. 8E经Sephadex LH-20、硅胶柱色谱纯化,得到化合物1(4 mg)和2(2 mg)。Fr. 10经硅胶柱色谱(200~300目)分离,氯仿-甲醇(20∶1→0∶1)梯度洗脱,得9个流分(Fr. 10A~10I)。Fr. 10F经反复硅胶、ODS和Sephadex LH-20柱色谱分离,得化合物3(200 mg)、4(50 mg)和5(20 mg)。Fr. 10G经Sephadex LH-20柱色谱分离,得化合物6(55 mg)和7(15 mg)。
3 结构鉴定
化合物1:比旋光度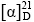 -83° (c 0.1,MeOH)。
-83° (c 0.1,MeOH)。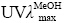 (nm): 326,261,211。HR-ESI-MS给出准分子离子峰m/z 567.112 6 [M-H]-,计算值567.114 4,结合13C-NMR谱数据确定该化合物的分子式为C28H24O13。IR谱显示该化合物含羟基(3 440 cm-1)、羰基(1 658 cm-1)及苯基(1 619 cm-1和1 508 cm-1)等官能团。在化合物1的1D NMR中,出现1组对羟基苯甲酰基信号δH 7.83 (2H,d,J = 8.0 Hz,H-2′′′,6′′′),6.88 (2H,d,J = 8.0 Hz,H-3′′′,5′′′);δC 165.8 (C=O),162.5 (C-4′′′),131.9 (C-2′′′,6′′′),115.8 (C-3′′′,5′′′),1组葡萄糖残基信号δH4.98 (1H,d,J = 7.0 Hz,H-1″);δC101.4 (C-1″),73.7 (C-2″),76.2 (C-3″),70.7 (C-4″),74.5 (C-5″),64.1 (C-6″) 和1组木犀草素的信号 δH 6.73 (1H,s,H-3),6.49 (1H,d,J = 1.5 Hz,H-8),6.21 (1H,d,J = 2.0 Hz,H-6),7.46 (1H,d,J = 2.0 Hz,H-2′),7.25 (1H,dd,J = 8.5,2.0 Hz,H-6′),7.18 (1H,d,J = 8.5 Hz,H-5′)。提示化合物1是1 个木犀草素葡萄糖苷类化合物[8]。将化合物1和木犀草素- 7-O-(6″-O-对羟基苯甲酰基)-β-D-葡萄糖苷(2)的NMR数据[8]进行比较,发现二者数据接近。在化合物1的HMBC谱中,葡萄糖的H-1″ (δH 4.98) 与木犀草素的C-4′ (δC 148.7) 存在氢-碳远程相关;在NOE实验中,照射葡萄糖的H-1″ (δH 4.98) 时,木犀草素的H-5′ (δH 7.18) 信号发生明显增益。上述数据证明化合物1中葡萄糖的1位连接在木犀草素的C-4′位而不是C-7位。在1D NMR谱中,葡萄糖的H-6″ (δH 4.54,4.25) 和C-6″ (δC 64.1) 明显向低场位移,推测葡萄糖的6-OH被酰化,由此推断对羟基苯甲酰基连接在葡萄糖的6位;根据HMBC谱中葡萄糖H-6″b (δH 4.25) 与对羟基苯甲酰基的羰基C-7′′′ (δC 165.8) 的氢-碳远程相关信号进一步证实了上述推断。综上所述,化合物1的结构鉴定为木犀草素-4′-O-(6″-O-对羟基苯甲酰基)-β-D-葡萄糖苷,命名为牡荆宁G。结构见图 1,其核磁数据归属见表 1。
(nm): 326,261,211。HR-ESI-MS给出准分子离子峰m/z 567.112 6 [M-H]-,计算值567.114 4,结合13C-NMR谱数据确定该化合物的分子式为C28H24O13。IR谱显示该化合物含羟基(3 440 cm-1)、羰基(1 658 cm-1)及苯基(1 619 cm-1和1 508 cm-1)等官能团。在化合物1的1D NMR中,出现1组对羟基苯甲酰基信号δH 7.83 (2H,d,J = 8.0 Hz,H-2′′′,6′′′),6.88 (2H,d,J = 8.0 Hz,H-3′′′,5′′′);δC 165.8 (C=O),162.5 (C-4′′′),131.9 (C-2′′′,6′′′),115.8 (C-3′′′,5′′′),1组葡萄糖残基信号δH4.98 (1H,d,J = 7.0 Hz,H-1″);δC101.4 (C-1″),73.7 (C-2″),76.2 (C-3″),70.7 (C-4″),74.5 (C-5″),64.1 (C-6″) 和1组木犀草素的信号 δH 6.73 (1H,s,H-3),6.49 (1H,d,J = 1.5 Hz,H-8),6.21 (1H,d,J = 2.0 Hz,H-6),7.46 (1H,d,J = 2.0 Hz,H-2′),7.25 (1H,dd,J = 8.5,2.0 Hz,H-6′),7.18 (1H,d,J = 8.5 Hz,H-5′)。提示化合物1是1 个木犀草素葡萄糖苷类化合物[8]。将化合物1和木犀草素- 7-O-(6″-O-对羟基苯甲酰基)-β-D-葡萄糖苷(2)的NMR数据[8]进行比较,发现二者数据接近。在化合物1的HMBC谱中,葡萄糖的H-1″ (δH 4.98) 与木犀草素的C-4′ (δC 148.7) 存在氢-碳远程相关;在NOE实验中,照射葡萄糖的H-1″ (δH 4.98) 时,木犀草素的H-5′ (δH 7.18) 信号发生明显增益。上述数据证明化合物1中葡萄糖的1位连接在木犀草素的C-4′位而不是C-7位。在1D NMR谱中,葡萄糖的H-6″ (δH 4.54,4.25) 和C-6″ (δC 64.1) 明显向低场位移,推测葡萄糖的6-OH被酰化,由此推断对羟基苯甲酰基连接在葡萄糖的6位;根据HMBC谱中葡萄糖H-6″b (δH 4.25) 与对羟基苯甲酰基的羰基C-7′′′ (δC 165.8) 的氢-碳远程相关信号进一步证实了上述推断。综上所述,化合物1的结构鉴定为木犀草素-4′-O-(6″-O-对羟基苯甲酰基)-β-D-葡萄糖苷,命名为牡荆宁G。结构见图 1,其核磁数据归属见表 1。
 | 图 1 化合物1的结构式和关键的NOE、HMBC相关Fig.1 Structure and key NOE and HMBC correlations of compound 1 |
| 表 1 化合物1和2的NMR数据 Table 1NMR spectroscopic data of compounds 1 and 2 |
化合物2:黄色粉末,ESI-MS m/z: 567 [M-H]-。1H-NMR (500 MHz,DMSO-d6) δ: 13.02 (1H,s,5-OH),7.79 (2H,d,J = 8.0 Hz,H-2′′′,6′′′),7.42 (1H,d,J = 2.0 Hz,H-2′),7.41 (1H,dd,J = 8.0,2.0 Hz,H-6′),6.89 (1H,d,J = 8.0 Hz,H-5′),6.77 (1H,d,J = 2.0 Hz,H-8),6.75 (1H,s,H-3),6.71 (2H,d,J = 8.0 Hz,H-3′′′,5′′′),6.51 (1H,d,J = 2.0 Hz,H-6),5.20 (1H,d,J = 7.5 Hz,H-1″),4.56 (1H,d,J = 11.0 Hz,H-6″a),4.13 (1H,dd,J = 12.0,7.5 Hz,H-6″b),3.91(1H,d,J = 10.0 Hz,H-5″),3.45 (1H,m,H-2″),3.45 (1H,m,H-3″),3.32 (1H,m,H-4″);13C-NMR (125 MHz,DMSO-d6) 数据见表 1。以上数据与文献报道基本一致[8],故鉴定化合物2为木犀草素-7-O-(6″-O-对羟基苯甲酰基)-β-D-葡萄糖苷。
化合物3:黄色粉末,ESI-MS m/z: 609 [M-H]-。1H-NMR (500 MHz,CD3OD) δ: 7.51 (1H,d,J = 16.0 Hz,H-7′′′),7.23 (1H,d,J = 2.0 Hz,H-2′),7.21 (1H,dd,J = 8.0,2.0 Hz,H-6′),6.96 (1H,d,J = 2.0 Hz,H-2′′′),6.84 (1H,dd,J = 8.0,2.0 Hz,H-6′′′),6.81 (1H,d,J = 8.0 Hz,H-5′′′),6.72 (1H,d,J = 8.0 Hz,H-5′),6.37 (1H,s,H-3),6.34 (1H,s,H-8),6.23 (1H,d,J = 16.0 Hz,H-8′′′),4.96 (1H,d,J = 10.0 Hz,H-1″),4.57 (1H,d,J = 11.5 Hz,H-6″a),4.41 (1H,dd,J = 12.0,5.5 Hz,H-6″b),4.27 (1H,m,H-2″),3.73 (1H,brs,H-5″),3.58 (1H,m,H-4″),3.58 (1H,m,H-3″);13C-NMR (125 MHz,CD3OD) δ: 183.8 (C-4),169.4 (C-9′′′),166.0 (C-2),164.8 (C-7),162.0 (C-5),158.6 (C-9),150.8 (C-4′),149.5 (C-4′′′),147.2 (C-7′′′),146.8 (C-3′′′),146.6 (C-3′),127.8 (C-1′′′),123.5 (C-1′),123.1 (C-6′′′),120.5 (C-6′),116.8 (C-5′),116.5 (C-5′′′),115.3 (C-2′′′),114.9 (C-8′′′),114.2 (C-2′),108.6 (C-6),105.2 (C-10),103.9 (C-3),95.4 (C-8),79.9 (C-3″),79.9 (C-5″),75.5 (C-1″),72.6 (C-2″),72.0 (C-4″),65.1 (C-6″)。以上数据与文献报道基本一致[8],故鉴定化合物3为木犀草素-6-C-(6″-O-反式-咖啡酰基)-β-D-葡萄糖苷。
化合物4:黄色粉末,ESI-MS m/z: 609 [M-H]-。1H-NMR (500 MHz,CD3OD) δ: 7.36 (1H,d,J = 15.5 Hz,H-7′′′),7.25 (1H,d,J = 2.0 Hz,H-2′),7.22 (1H,dd,J = 8.5,2.5 Hz,H-6′),6.89 (1H,d,J = 2.0 Hz,H-2′′′),6.82 (1H,dd,J = 8.0,2.0 Hz,H-6′′′),6.78 (1H,d,J = 8.0 Hz,H-5′′′),6.68 (1H,d,J = 8.5 Hz,H-5′),6.40 (1H,s,H-8),6.38 (1H,s,H-3),6.04 (1H,d,J = 15.5 Hz,H-8′′′),5.66 (1H,brs,H-2″),5.12 (1H,d,J = 10.0 Hz,H-1″),3.96 (1H,dd,J = 12.0,2.0 Hz,H-6″a),3.82 (1H,dd,J = 12.0,5.5 Hz,H-6″b),3.77 (1H,t,J = 9.0 Hz,H-3″),3.63 (1H,t,J = 9.0 Hz,H-4″),3.53 (1H,m,H-5″);13C-NMR (125 MHz,CD3OD) δ: 183.9 (C-4),168.4 (C-9′′′),166.3 (C-2),164.6 (C-7),161.5 (C-5),158.8 (C-9),151.0 (C-4′),149.5 (C-4′′′),147.1 (C-7′′′),146.9 (C-3′),146.7 (C-3′′′),128.0 (C-1′′′),123.6 (C-1′),123.0 (C-6′′′),120.5 (C-6′),116.8 (C-5′),116.6 (C-5′′′),115.2 (C-2′′′),115.0 (C-8′′′),114.3 (C-2′),107.9 (C-6),105.1 (C-10),104.0 (C-3),95.5 (C-8),82.9 (C-5″),78.1 (C-3″),74.0 (C-2″),73.3 (C-1″),71.9 (C-4″),62.9 (C-6″)。以上数据与文献报道基本一致[8],故鉴定化合物4为木犀草素-6-C -(2″- O-反式-咖啡酰基)-β-D-葡萄糖苷。
化合物5:黄色粉末,ESI-MS m/z: 567 [M-H]-。1H-NMR (500 MHz,DMSO-d6) δ: 13.59 (1H,s,5-OH),7.80 (2H,d,J = 8.0 Hz,H-2′′′,6′′′),7.40 (1H,dd,J = 8.5,2.0 Hz,H-6′),7.39 (1H,d,J = 2.0 Hz,H-2′),6.88 (1H,d,J = 8.5 Hz,H-5′),6.84 (2H,d,J = 8.0 Hz,H-3′′′,5′′′),6.65 (1H,s,H-3),6.47 (1H,s,H-8),4.65 (1H,d,J = 9.5 Hz,H-1″),4.43 (1H,d,J = 11.5 Hz,H-6″a),4.25 (1H,dd,J = 11.5,4.5 Hz,H-6″b),4.13 (1H,t,J = 8.0 Hz,H-2″),3.50 (1H,brs,H-5″),3.32 (1H,t,J = 9.0 Hz,H-4″),3.26 (1H,t,J = 8.5 Hz,H-3″);13C-NMR (125 MHz,DMSO-d6) δ: 181.8 (C-4),165.5 (C-7′′′),163.6 (C-2),163.3 (C-7),161.9 (C-4′′′),160.8 (C-5),156.2 (C-9),149.7 (C-4′),145.7 (C-3′),131.4 (C-2′′′,6′′′),121.3 (C-1′),120.4 (C-1′′′),118.9 (C-6′),116.0 (C-5′),115.3 (C-3′′′,5′′′),113.2 (C-2′),108.6 (C-6),103.3 (C-10),102.7 (C-3),93.5 (C-8),78.7 (C-3″),78.1 (C-5″),73.2 (C-1″),70.2 (C-2″),70.0 (C-4″),64.2 (C-6″)。以上数据与文献报道基本一致[9],故鉴定化合物5为perfoliatumin A。
化合物6:黄色粉末,ESI-MS m/z: 431 [M-H]-。1H-NMR (500 MHz,DMSO-d6) δ: 13.58 (1H,s,5-OH),7.87 (2H,d,J = 8.5 Hz,H-2′,6′),6.92 (2H,d,J = 8.5 Hz,H-3′,5′),6.73 (1H,s,H-8),6.54 (1H,s,H-3),4.66 (1H,d,J = 9.5 Hz,H-1″),4.10 (1H,m,H-2″),3.74 (1H,d,J = 11.0 Hz,H-6″a),3.50 (1H,dd,J = 10.5,4.5 Hz,H-6″b),3.29~3.18 (3H,m,H-3″~5″);13C-NMR (125 MHz,DMSO-d6) δ: 182.1 (C-4),163.8 (C-2),163.5 (C-7),161.3 (C-4′),160.8 (C-5),156.5 (C-9),128.6 (C-2′,6′),121.3 (C-1′),116.2 (C-3′,5′),108.9 (C-6),103.7 (C-10),103.0 (C-3),94.0 (C-8),81.7 (C-5″),79.1 (C-3″),73.3 (C-1″),70.8 (C-2″),70.5 (C-4″),61.7 (C-6″)。以上数据与文献报道基本一致[6],故鉴定化合物6为异牡荆素。
化合物7:黄色粉末,ESI-MS m/z: 447 [M-H]-。1H-NMR (500 MHz,DMSO-d6) δ: 7.44 (1H,dd,J = 8.0,1.5 Hz,H-6′),7.41 (1H,d,J = 1.5 Hz,H-2′),6.91 (1H,d,J = 8.0 Hz,H-5′),6.79 (1H,d,J = 1.5 Hz,H-8),6.74 (1H,s,H-3),6.44 (1H,d,J = 1.5 Hz,H-6),5.08 (1H,d,J = 7.5 Hz,H-1″),3.72~3.19 (6H,m,H-2″~6″);13C-NMR (125 MHz,DMSO-d6) δ: 181.9 (C-4),164.5 (C-2),162.9 (C-7),161.1 (C-5),156.9 (C-9),150.0 (C-4′),146.0 (C-3′),121.3 (C-1′),119.2 (C-6′),116.0 (C-5′),113.5 (C-2′),105.3 (C-10),103.1 (C-3),99.9 (C-6),99.5 (C-1″),94.7 (C-8),77.2 (C-3″),76.4 (C-5″),73.1 (C-2″),69.6 (C-4″),60.6 (C-6″)。以上数据与文献报道基本一致[10],故鉴定化合物7为木犀草素-7-O-β-D-葡萄糖苷。
| [1] | 中国药典 [S]. 一部. 2010. |
| [2] | 国家中医药管理局中华本草编委会. 中华本草 (第18卷) [M]. 上海: 上海科技出版社, 1999. |
| [3] | Taguchi H. Studies on the constituents of Vitex cannabifolia [J]. Chem Pharm Bull, 1976, 24(7): 1668-1670. |
| [4] | Chen Y J, Li C M, Ling W W, et al. A rearranged labdane-type diterpenoid and other constituents from Vitex negundo var. cannabifolia [J]. Biochem Syst Ecol, 2012, 40(2): 98-102. |
| [5] | Yamasaki T, Kawabata T, Masuoka C, et al. Two new lignan glucosides from the fruit of Vitex cannabifolia [J]. J Nat Med, 2008, 62(1): 47-51. |
| [6] | Ling T J, Ling W W, Chen Y J, et al. Antiseptic activity and phenolic constituents of the aerial parts of Vitex negundo var. cannabifolia [J]. Molecules, 2010, 15(11): 8469-8477. |
| [7] | Li M M, Su X Q, Sun J, et al. Anti-inflammatory ursane- and oleanane-type triterpenoids from Vitex negundo var. cannabifolia [J]. J Nat Prod, 2014, 77(10): 2248-2254. |
| [8] | Hirobe C, Qiao Z S, Takey K, et al. Cytotoxic flavonoids from Vitex agnus-castus [J]. Phytochemistry, 1997, 46(3): 521-524. |
| [9] | Zhu G H, Wang D Y, Jurrcai M. New compounds from Polygonum perfoliatum L. [J]. Indian J Heterocy Ch, 2000, 10(1): 41-44. |
| [10] | 徐燕, 梁敬钰. 苦苣菜的化学成分 [J]. 中国药科大学学报, 2005, 36(5): 411-413. |
 2015, Vol. 46
2015, Vol. 46


