甘草Glycyrrhizae Radix et Rhizoma是豆科(Leguminosae)甘草属Glycyrrhiza Linn. 植物的干燥根和根茎,始载于《神农本草经》,为我国传统中药,有“十方九草”之美誉,被大量应用于临床配方;被《中国药典》2010年版收录为甘草Glycyrrhiza uralensis Fisch.、胀果甘草Glycyrrhiza inflata Bat. 和光果甘草Glycyrrhiza glabra L. 的干燥根和根茎。其中甘草分布最广,主要分布于中国的华北、东北和西北地区,胀果甘草分布于新疆及甘肃西北部,而光果甘草仅产于我国新疆地区。现有研究表明,甘草主要化学成分为三萜和黄酮类,此外,还含有生物碱、香豆素等。为了更深入地研究甘草的物质基础,本实验对甘草的化学成分进行了研究,从甘草95%乙醇提取物正丁醇萃取部分分离得到14个化合物,包括7个三萜皂苷和7个黄酮类成分,分别鉴定为macedonoside E(1)、22β-乙酰基乌拉尔甘草皂苷C(22β-acetyl-uralsaponin C,2)、甘草酸(glycyrrhizic acid,3)、乌拉尔甘草皂苷F(uralsaponin F,4)、甘草皂苷G2(licorice-saponin G2,5)、22β-乙酰基甘草醛(22β-acetoxyl-glycyrrhal-dehyde,6)、甘草酸甲酯(3β-O-[β-D-(6-methyl)- glucuronopyranosyl-(1→2)-β-D-glucuronopyranosyl]- glycyrrhizic acid,7)、甘草素(liquiritigenin,8)、柚皮素(naringenin,9)、异甘草素(isoliquiritigenin,10)、芒柄花苷(ononin,11)、甘草苷(liquiritin,12)、异佛来心苷(isoviolanthin,13)、芹糖甘草苷(liquiritin apioside,14)。其中化合物1和2为2个新三萜皂苷类化合物。
1 仪器与材料200 L提取罐(浙江苍南县立瓯石化设备有限公司),减压蒸馏器(浙江苍南县立瓯石化设备有限公司);Bruker AV-500型核磁共振仪(Bruker BioSpin公司);Buchi688型中压液相色谱系统(BÜCHI,瑞士);Agilent 1100型制备液相色谱仪(Agilent,美国),制备用HPLC色谱柱为Zorbax SB-C18(250 mm×21.2 mm,7 μm);Agilent1100高效液相色谱仪,配在线真空脱气机、四元梯度泵、自动进样器、柱温箱、G1315B-DAD检测器,G1314A-VWD检测器(Agilent,美国),分析用色谱柱为Zorbax SB-C18(250 mm×4.6 mm,5 μm);Finnigan LCQ Daca XPplus离子阱质谱仪(Thermo Fisher,美国),Thermo Finnigan Xcalibur 1.3工作站;R-200旋转蒸发仪(BÜCHI,瑞士);Minispin离心机(Eppendorf,德国);KQ-250B型超声仪(40 KHz,昆山市超声仪器有限公司);色谱纯乙腈(Merck,德国)、甲酸(ROE Scientific Inc.,美国);超纯水(Millipore,美国);柱色谱硅胶(100~200、200~300、300~400目)、GF254薄层色谱硅胶板(青岛海洋化工厂);分析纯石油醚、醋酸乙酯及甲醇(浙江常青化工有限公司)。
甘草饮片购自于浙江中医药大学中药饮片厂,产自甘肃陇西,经浙江大学药学院陈柳蓉副教授鉴定为豆科甘草属植物甘草Glycyrrhiza uralensis Fisch. 的根和根茎,原生药样本(GC-120509)存放于浙江大学药物信息学研究所。
2 提取与分离甘草饮片20 kg,用8倍量的95%乙醇回流提取3次,每次2 h,将所得提取物回收至无醇味后,得约4 L浓缩液,依次用等体积的石油醚、醋酸乙酯、正丁醇各萃取3次,得到石油醚萃取物19 g,醋酸乙酯萃取物347 g,正丁醇萃取物409 g。将正丁醇部分进行硅胶柱色谱,用二氯甲烷-甲醇不同比例(100∶1→0∶1)进行梯度洗脱,经薄层色谱分析后,合并相同流分,得到4个部分。二氯甲烷-甲醇(1∶1)洗脱部分再经ODS柱色谱,分别用10%和20%乙腈水洗脱,得到化合物1(4.1 mg)和2(5.5 mg);其余各部分经多次中压硅胶柱色谱、ODS柱色谱以及高效液相制备色谱等手段进一步分离纯化,得到化合物3(11.3 mg)、4(5.1 mg)、5(31.3 mg)、6(12.9 mg)、7(190.0 mg)、8(104.0 mg)、9(11.0 mg)、10(179.0 mg)、11(15.9 mg)、12(136.1 mg)、13(3.6 mg)、14(14.3 mg)。
3 结构鉴定
化合物1:白色固体。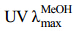 (nm): 254。Q-TOF-MS分析给出m/z 837.392 8 [M-H]-,839.406 0 [M+H]+,其分子式为C42H62O17。其1H-NMR (500 MHz,CD3OD) 在δH 0.81 (3H,s,H-28),0.83 (3H,s,H-24),1.06 (3H,s,H-23),1.12 (3H,s,H-26),1.13 (3H,s,H-25),1.19 (3H,s,H-29) 和1.42 (3H,s,H-27) 显示有7个甲基单峰信号,这是齐墩果烷型三萜类化合物的特征。1H-NMR中2个糖端基质子信号δH4.51 (1H,d,J = 7.4 Hz,H-1′) 和δH4.62 (1H,d,J = 7.7 Hz,H-1″) 及端基碳信号δC105.4 (C-1′),106.4 (C-1″) 与甘草酸(化合物3)中的2个葡萄糖醛酸β-D-GlcA-(2→1)-β-D-GlcA-端基质子和端基碳的化学位移及耦合常数基本一致,结合分子式,可推测该化合物为连有2个β-D-葡萄糖醛酸的三萜皂苷;HMBC谱中δH 4.51 (1H,d,J = 7.4 Hz,H-1′) 与δC 90.9 (C-3) 的相关信号表明糖基连接在三萜苷元的C-3位,HMBC谱中δH 4.62 (1H,d,J = 7.7 Hz,H-1″) 与δC 84.1 (C-2′) 的相关信号证明2个葡萄糖醛酸的链接顺序为β-D-GlcA-(2→1)-β- D-GlcA。双键质子信号δH 5.58 (1H,s,H-12)、双键次甲基碳信号δC 129.1 (C-12)、季碳信号173.2 (C-13) 及羰基碳信号δC 203.0 (C-11) 表明该化合物结构为11-oxo,Δ12-齐墩果烷型三萜[1]。δC 180.1 (C-30) 表明结构中有1个羧基,且HMBC谱(图 1)中可见δH 0.81 (3H,s,H-29) 与δC 37.8 (C-19),71.0 (C-21),180.1 (C-30) 的相关信号;研究发现,如果C-30位为甲基,则NOESY谱(图 1)中可见H-18(β) 和C-30位甲基的相关信号,在化合物1的NOESY谱中有δH 2.21 (1H,d,J = 13.4 Hz,H-18) 与δH 0.81 (3H,s,H-28) 的相关信号,表明H-18为β-H,但未见有δH 2.21 (1H,d,J = 13.4 Hz,H-18) 与δH 1.19 (3H,s,H-29) 的相关信号,表明该齐墩果烷型三萜的C-30为羧基、C-29为甲基。NOESY谱中δH 3.18 (1H,dd,J = 11.6,4.4 Hz,H-3) 与1.06 (3H,s,H-23)的相关信号、δH4.10 (1H,brs,H-21) 与0.81 (3H,s,H-29) 的相关信号证明H-3与H-21均为α-H。综上所述,确定化合物1的结构见图 1,1H-、13C-NMR及DEPT谱数据见表 1,与化合物macedonoside A的13C-NMR数据比较,表明该化合物与macedonoside A的结构相近[1]。经查询,在CA中未见有该化合物结构的报道,该化合物为一新化合物,命名为macedonoside E。
(nm): 254。Q-TOF-MS分析给出m/z 837.392 8 [M-H]-,839.406 0 [M+H]+,其分子式为C42H62O17。其1H-NMR (500 MHz,CD3OD) 在δH 0.81 (3H,s,H-28),0.83 (3H,s,H-24),1.06 (3H,s,H-23),1.12 (3H,s,H-26),1.13 (3H,s,H-25),1.19 (3H,s,H-29) 和1.42 (3H,s,H-27) 显示有7个甲基单峰信号,这是齐墩果烷型三萜类化合物的特征。1H-NMR中2个糖端基质子信号δH4.51 (1H,d,J = 7.4 Hz,H-1′) 和δH4.62 (1H,d,J = 7.7 Hz,H-1″) 及端基碳信号δC105.4 (C-1′),106.4 (C-1″) 与甘草酸(化合物3)中的2个葡萄糖醛酸β-D-GlcA-(2→1)-β-D-GlcA-端基质子和端基碳的化学位移及耦合常数基本一致,结合分子式,可推测该化合物为连有2个β-D-葡萄糖醛酸的三萜皂苷;HMBC谱中δH 4.51 (1H,d,J = 7.4 Hz,H-1′) 与δC 90.9 (C-3) 的相关信号表明糖基连接在三萜苷元的C-3位,HMBC谱中δH 4.62 (1H,d,J = 7.7 Hz,H-1″) 与δC 84.1 (C-2′) 的相关信号证明2个葡萄糖醛酸的链接顺序为β-D-GlcA-(2→1)-β- D-GlcA。双键质子信号δH 5.58 (1H,s,H-12)、双键次甲基碳信号δC 129.1 (C-12)、季碳信号173.2 (C-13) 及羰基碳信号δC 203.0 (C-11) 表明该化合物结构为11-oxo,Δ12-齐墩果烷型三萜[1]。δC 180.1 (C-30) 表明结构中有1个羧基,且HMBC谱(图 1)中可见δH 0.81 (3H,s,H-29) 与δC 37.8 (C-19),71.0 (C-21),180.1 (C-30) 的相关信号;研究发现,如果C-30位为甲基,则NOESY谱(图 1)中可见H-18(β) 和C-30位甲基的相关信号,在化合物1的NOESY谱中有δH 2.21 (1H,d,J = 13.4 Hz,H-18) 与δH 0.81 (3H,s,H-28) 的相关信号,表明H-18为β-H,但未见有δH 2.21 (1H,d,J = 13.4 Hz,H-18) 与δH 1.19 (3H,s,H-29) 的相关信号,表明该齐墩果烷型三萜的C-30为羧基、C-29为甲基。NOESY谱中δH 3.18 (1H,dd,J = 11.6,4.4 Hz,H-3) 与1.06 (3H,s,H-23)的相关信号、δH4.10 (1H,brs,H-21) 与0.81 (3H,s,H-29) 的相关信号证明H-3与H-21均为α-H。综上所述,确定化合物1的结构见图 1,1H-、13C-NMR及DEPT谱数据见表 1,与化合物macedonoside A的13C-NMR数据比较,表明该化合物与macedonoside A的结构相近[1]。经查询,在CA中未见有该化合物结构的报道,该化合物为一新化合物,命名为macedonoside E。
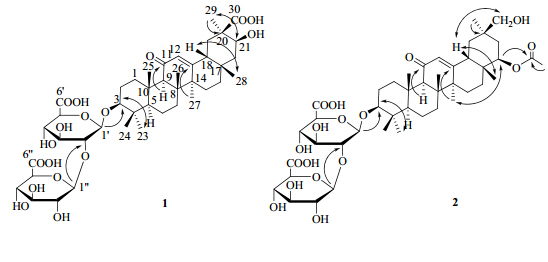 | 图 1 化合物1和2的结构及重要相关关系Fig.1 Structures and key correlations of compounds 1 and 2 |
化合物2:白色固体。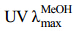 (nm): 254。Q-TOF-MS分析给出其分子式为C44H66O17,其正离子模式下为m/z 889.421 5 [M+Na]+。与化合物1类似,在化合物2的1H-NMR谱中的高场可见齐墩果烷型三萜特征的7个甲基单峰质子信号δH0.71 (3H,s,H-24),0.76 (3H,s,H-28),0.84 (3H,s,H-29),0.94 (3H,s,H-23),1.03 (6H,s,H-25,26) 和1.34 (3H,s,H-27),此外,在δH1.98 (3H,s) 有1个甲基单峰信号;2个糖端基质子信号δH 4.32 (1H,d,J = 7.4 Hz,H-1′),4.45 (1H,d,J = 7.7 Hz,H-1″) 及端基碳信号δC103.5 (C-1′),104.3 (C-1″),与22β-乙酰基甘草醛(6)中2个葡萄糖醛酸β-D-GlcA-(2→1)-β-D-GlcA-端基质子及端基碳的化学位移和耦合常数基本一致,表明化合物2中含有2个β-D-GlcA。1H-和13C-NMR中显示有α,β-不饱和酮的质子信号和碳信号:δH5.55 (s,H-12) 和δC 199.1 (C-11),127.7 (C-12),168.5 (C-13);13C-NMR中除了糖基上连氧碳信号外,还有2个连氧次甲基碳信号δC88.0 (C-3),76.6 (C-22)、1个连氧亚甲基碳信号δC66.3 (C-30),这些与uralsaponin C的13C-NMR数据相似[2];另外,碳谱上还可见羰基碳信号δC169.8,而且HMBC谱中δC 169.8与δH 1.98 (3H,s) 和δH4.53 (1H,brs,H-22) 均有相关信号,表明C-22位上连有1个乙酰氧基。δC 88.0 (C-3) 与δH 4.32 (1H,d,J = 7.4 Hz,H-1′) 的相关峰及δC 82.1 (C-2′) 与δH 4.45 (1H,d,J = 7.7 Hz,H-1″) 的相关信号表明糖基位于苷元C-3位,2个β-D-GlcA为2→1连接。NOESY谱中δH 3.03 (H-3,*表示与其他信号有重叠) 与δH 0.94 (3H,s,H-23) 的相关信号表明H-3为α构型;δH 4.53 (1H,brs,H-22),1.34 (3H,s,H-27) 的相关信号证明H-22为α构型;δH 2.27 (1H,brd,J = 11.7 Hz,H-18) 与0.76 (s,H-28) 的相关信号表明H-18为β-H,δH 2.27 (1H,brd,J = 11.7 Hz,H-18) 与3.23、3.36 (H-30) 的相关信号证明C-30为羟甲基,C-29为甲基。综上所述,化合物2的结构如图 1所示,1H-、13C-NMR数据见表 1。经查询,化合物2为一新化合物,该化合物结构比uralsaponin C多了1个乙酰基,故将其命名为22β-乙酰基乌拉尔甘草皂苷C。
(nm): 254。Q-TOF-MS分析给出其分子式为C44H66O17,其正离子模式下为m/z 889.421 5 [M+Na]+。与化合物1类似,在化合物2的1H-NMR谱中的高场可见齐墩果烷型三萜特征的7个甲基单峰质子信号δH0.71 (3H,s,H-24),0.76 (3H,s,H-28),0.84 (3H,s,H-29),0.94 (3H,s,H-23),1.03 (6H,s,H-25,26) 和1.34 (3H,s,H-27),此外,在δH1.98 (3H,s) 有1个甲基单峰信号;2个糖端基质子信号δH 4.32 (1H,d,J = 7.4 Hz,H-1′),4.45 (1H,d,J = 7.7 Hz,H-1″) 及端基碳信号δC103.5 (C-1′),104.3 (C-1″),与22β-乙酰基甘草醛(6)中2个葡萄糖醛酸β-D-GlcA-(2→1)-β-D-GlcA-端基质子及端基碳的化学位移和耦合常数基本一致,表明化合物2中含有2个β-D-GlcA。1H-和13C-NMR中显示有α,β-不饱和酮的质子信号和碳信号:δH5.55 (s,H-12) 和δC 199.1 (C-11),127.7 (C-12),168.5 (C-13);13C-NMR中除了糖基上连氧碳信号外,还有2个连氧次甲基碳信号δC88.0 (C-3),76.6 (C-22)、1个连氧亚甲基碳信号δC66.3 (C-30),这些与uralsaponin C的13C-NMR数据相似[2];另外,碳谱上还可见羰基碳信号δC169.8,而且HMBC谱中δC 169.8与δH 1.98 (3H,s) 和δH4.53 (1H,brs,H-22) 均有相关信号,表明C-22位上连有1个乙酰氧基。δC 88.0 (C-3) 与δH 4.32 (1H,d,J = 7.4 Hz,H-1′) 的相关峰及δC 82.1 (C-2′) 与δH 4.45 (1H,d,J = 7.7 Hz,H-1″) 的相关信号表明糖基位于苷元C-3位,2个β-D-GlcA为2→1连接。NOESY谱中δH 3.03 (H-3,*表示与其他信号有重叠) 与δH 0.94 (3H,s,H-23) 的相关信号表明H-3为α构型;δH 4.53 (1H,brs,H-22),1.34 (3H,s,H-27) 的相关信号证明H-22为α构型;δH 2.27 (1H,brd,J = 11.7 Hz,H-18) 与0.76 (s,H-28) 的相关信号表明H-18为β-H,δH 2.27 (1H,brd,J = 11.7 Hz,H-18) 与3.23、3.36 (H-30) 的相关信号证明C-30为羟甲基,C-29为甲基。综上所述,化合物2的结构如图 1所示,1H-、13C-NMR数据见表 1。经查询,化合物2为一新化合物,该化合物结构比uralsaponin C多了1个乙酰基,故将其命名为22β-乙酰基乌拉尔甘草皂苷C。
化合物3:白色胶状固体。ESI-MS m/z: 821 [M-H]-,823 [M+H]+;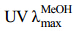 (nm): 254。1H-NMR (500 MHz,CD3OD) δ: 5.57 (1H,s,H-12),4.63 (1H,d,J = 7.7 Hz,H-1″),4.52 (1H,d,J = 7.4 Hz,H-1′),2.44 (1H,s,H-9),2.19 (1H,dd,J = 13.4,3.2 Hz,H-18),1.42 (3H,s,H-27),1.17 (3H,s,H-29),1.13 (6H,s,H-25,26),1.06 (3H,s,H-23),0.83 (6H,s,H-24,28);13C-NMR (125 MHz,CD3OD) 数据见表 2。以上数据与文献报道一致[3, 4],故鉴定化合物3为甘草酸。
(nm): 254。1H-NMR (500 MHz,CD3OD) δ: 5.57 (1H,s,H-12),4.63 (1H,d,J = 7.7 Hz,H-1″),4.52 (1H,d,J = 7.4 Hz,H-1′),2.44 (1H,s,H-9),2.19 (1H,dd,J = 13.4,3.2 Hz,H-18),1.42 (3H,s,H-27),1.17 (3H,s,H-29),1.13 (6H,s,H-25,26),1.06 (3H,s,H-23),0.83 (6H,s,H-24,28);13C-NMR (125 MHz,CD3OD) 数据见表 2。以上数据与文献报道一致[3, 4],故鉴定化合物3为甘草酸。
化合物4:白色固体。ESI-MS m/z: 895 [M-H]-,897 [M+H]+;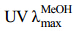 (nm): 254。1H-NMR (500MHz,CD3OD) δ: 5.65 (1H,s,H-12),4.83 (1H,d,J = 7.8 Hz,H-1″),4.55 (2H,s,H-1′,22),2.47 (1H,s,H-9),1.98 (3H,s,COCH3),1.46 (3H,s,H-27),1.20 (3H,s,H-23),1.17 (3H,s,H-29),1.14 (3H,s,H-26),1.08 (3H,s,H-25),0.85 (3H,s,H-28);13C-NMR (125 MHz,CD3OD) 数据见表 2。以上数据与文献报道一致[2],故鉴定化合物4为乌拉尔甘草皂苷F。
(nm): 254。1H-NMR (500MHz,CD3OD) δ: 5.65 (1H,s,H-12),4.83 (1H,d,J = 7.8 Hz,H-1″),4.55 (2H,s,H-1′,22),2.47 (1H,s,H-9),1.98 (3H,s,COCH3),1.46 (3H,s,H-27),1.20 (3H,s,H-23),1.17 (3H,s,H-29),1.14 (3H,s,H-26),1.08 (3H,s,H-25),0.85 (3H,s,H-28);13C-NMR (125 MHz,CD3OD) 数据见表 2。以上数据与文献报道一致[2],故鉴定化合物4为乌拉尔甘草皂苷F。
| 表 1 化合物1 (CD3OD) 和2 (DMSO-d6) 的1H-NMR、13C-NMR及DEPT谱数据 Table 1 1H-NMR,13C-NMR,and DEPT data of compounds 1 (CD3OD) and 2 (DMSO-d6) |
| 表 2 化合物3~7的13C-NMR数据 (aCD3OD,bDMSO-d6, 125 MHz) Table 2 13C-NMR data of compounds 3—7 (aCD3OD,bDMSO-d6,125 MHz) |
化合物5:白色固体。ESI-MS m/z: 837 [M-H]-,839 [M+H]+;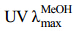 (nm): 254。1H-NMR (500 MHz,DMSO-d6) δ: 5.39 (1H,s,H-12),4.63 (1H,d,J = 7.7 Hz,H-1″),4.41 (1H,d,J = 7.3 Hz,H-1′),2.32 (1H,s,H-9),1.32 (3H,s,H-27),1.08 (3H,s,H-29),1.06 (3H,s,H-23),1.00 (6H,s,H-25,26),0.74 (3H,s,H-28);13C-NMR (125 MHz,DMSO-d6) 数据见表 2。以上数据与文献报道一致[5],故鉴定化合物5为甘草皂苷G2。
(nm): 254。1H-NMR (500 MHz,DMSO-d6) δ: 5.39 (1H,s,H-12),4.63 (1H,d,J = 7.7 Hz,H-1″),4.41 (1H,d,J = 7.3 Hz,H-1′),2.32 (1H,s,H-9),1.32 (3H,s,H-27),1.08 (3H,s,H-29),1.06 (3H,s,H-23),1.00 (6H,s,H-25,26),0.74 (3H,s,H-28);13C-NMR (125 MHz,DMSO-d6) 数据见表 2。以上数据与文献报道一致[5],故鉴定化合物5为甘草皂苷G2。
化合物6:白色固体。ESI-MS m/z: 863 [M-H]-,865 [M+H]+,865 [M+H]+,689 [M+H-Glc]+,513 [M+H-2Glc]+,495 [M+H-2Glc-H2O]+;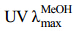 (nm): 254。1H-NMR (500 MHz,DMSO-d6) δ: 9.36 (1H,s,H-30),5.48 (1H,s,H-12),4.47 (1H,d,J = 7.7 Hz,H-1″),4.36 (1H,d,J = 7.4 Hz,H-1′),2.33 (1H,s,H-9),1.93 (3H,s,COCH3),1.40 (3H,s,H-27),1.02 (6H,s,H-25,26),0.94 (3H,s,H-23),0.90 (3H,s,H-29),0.70 (3H,s,H-24),0.67 (3H,s,H-28);13C-NMR (125 MHz,DMSO-d6) 见表 2。以上数据与文献报道一致[6],故鉴定化合物6为22β-乙酰基甘草醛。
(nm): 254。1H-NMR (500 MHz,DMSO-d6) δ: 9.36 (1H,s,H-30),5.48 (1H,s,H-12),4.47 (1H,d,J = 7.7 Hz,H-1″),4.36 (1H,d,J = 7.4 Hz,H-1′),2.33 (1H,s,H-9),1.93 (3H,s,COCH3),1.40 (3H,s,H-27),1.02 (6H,s,H-25,26),0.94 (3H,s,H-23),0.90 (3H,s,H-29),0.70 (3H,s,H-24),0.67 (3H,s,H-28);13C-NMR (125 MHz,DMSO-d6) 见表 2。以上数据与文献报道一致[6],故鉴定化合物6为22β-乙酰基甘草醛。
化合物7:白色固体。ESI-MS m/z: 835 [M-H]-,837 [M+H]+;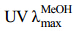 (nm): 252。1H-NMR (500 MHz,CD3OD) δ: 5.57 (1H,s,H-12),4.63 (1H,d,J = 7.8 Hz,H-1″),4.50 (1H,d,J = 7.6 Hz,H-1′),3.74 (3H,s,COOCH3),2.44 (1H,s,H-9),1.42 (3H,s,H-27),1.17 (3H,s,H-29),1.13 (6H,s,H-25,26),1.03 (3H,s,H-23),0.83 (3H,s,H-28),0.79 (3H,s,H-24);13C-NMR (125 MHz,CD3OD) 见表 2。以上数据与文献报道一致[2],故鉴定化合物7为甘草酸甲酯。
(nm): 252。1H-NMR (500 MHz,CD3OD) δ: 5.57 (1H,s,H-12),4.63 (1H,d,J = 7.8 Hz,H-1″),4.50 (1H,d,J = 7.6 Hz,H-1′),3.74 (3H,s,COOCH3),2.44 (1H,s,H-9),1.42 (3H,s,H-27),1.17 (3H,s,H-29),1.13 (6H,s,H-25,26),1.03 (3H,s,H-23),0.83 (3H,s,H-28),0.79 (3H,s,H-24);13C-NMR (125 MHz,CD3OD) 见表 2。以上数据与文献报道一致[2],故鉴定化合物7为甘草酸甲酯。
化合物8:白色针状结晶(甲醇)。ESI-MS m/z: 255 [M-H]-,511 [2M-H]-,135 [M-H-120]-(甘草素特征碎片);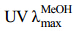 (nm): 236,274,318。1H-NMR (500 MHz,CD3OD) δ: 7.70 (1H,d,J = 8.7 Hz,H-5),7.29 (2H,d,J = 8.5 Hz,H-2′,6′),6.81 (2H,d,J = 8.6 Hz,H-3′,5′),6.48 (1H,dd,J = 8.7,2.3 Hz,H-6),6.34 (1H,d,J = 2.2 Hz,H-8),5.31 (1H,dd,J = 13.1,2.8 Hz,H-2),3.00 (1H,dd,J = 16.9,13.2 Hz,H-3),2.65 (1H,dd,J = 16.9,2.9 Hz,H-3);13C-NMR (125 MHz,CD3OD) δ: 193.5 (C-4),166.7 (C-7),165.5 (C-9),158.8 (C-4′),131.3 (C-1′),129.8 (C-5),129.0 (C-2′,6′),116.3 (C-3′,5′),114.9 (C-10),111.7 (C-6),103.8 (C-8),80.9 (C-2),44.8 (C-3)。以上数据与文献报道一致[7],故鉴定化合物8为甘草素。
(nm): 236,274,318。1H-NMR (500 MHz,CD3OD) δ: 7.70 (1H,d,J = 8.7 Hz,H-5),7.29 (2H,d,J = 8.5 Hz,H-2′,6′),6.81 (2H,d,J = 8.6 Hz,H-3′,5′),6.48 (1H,dd,J = 8.7,2.3 Hz,H-6),6.34 (1H,d,J = 2.2 Hz,H-8),5.31 (1H,dd,J = 13.1,2.8 Hz,H-2),3.00 (1H,dd,J = 16.9,13.2 Hz,H-3),2.65 (1H,dd,J = 16.9,2.9 Hz,H-3);13C-NMR (125 MHz,CD3OD) δ: 193.5 (C-4),166.7 (C-7),165.5 (C-9),158.8 (C-4′),131.3 (C-1′),129.8 (C-5),129.0 (C-2′,6′),116.3 (C-3′,5′),114.9 (C-10),111.7 (C-6),103.8 (C-8),80.9 (C-2),44.8 (C-3)。以上数据与文献报道一致[7],故鉴定化合物8为甘草素。
化合物9:黄色固体粉末。ESI-MS m/z: 271 [M-H]-,543 [2M-H]-;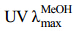 (nm): 238,278,322。1H-NMR (500 MHz,CD3OD) δ: 7.31 (2H,d,J = 8.5 Hz,H-2′,6′),6.82 (2H,d,J = 8.6 Hz,H-3′,5′),5.89 (1H,d,J = 1.9 Hz,H-8),5.88 (1H,d,J = 1.9 Hz,H-6),5.32 (1H,dd,J = 12.9,2.9 Hz,H-2),3.10 (1H,dd,J = 17.1,13.0 Hz,H-3),2.68 (1H,dd,J = 17.1,3.0 Hz,H-3);13C-NMR (125 MHz,CD3OD) δ: 197.7 (C-4),168.7 (C-7),165.5 (C-5),164.9 (C-9),159.0 (C-4′),131.4 (C-1′),129.0 (C-2′,6′),116.3 (C-3′,5′),103.3 (C-10),97.2 (C-6),96.3 (C-8),80.5 (C-2),44.0 (C-3)。以上数据与文献报道一致[8],故鉴定化合物9为柚皮素。
(nm): 238,278,322。1H-NMR (500 MHz,CD3OD) δ: 7.31 (2H,d,J = 8.5 Hz,H-2′,6′),6.82 (2H,d,J = 8.6 Hz,H-3′,5′),5.89 (1H,d,J = 1.9 Hz,H-8),5.88 (1H,d,J = 1.9 Hz,H-6),5.32 (1H,dd,J = 12.9,2.9 Hz,H-2),3.10 (1H,dd,J = 17.1,13.0 Hz,H-3),2.68 (1H,dd,J = 17.1,3.0 Hz,H-3);13C-NMR (125 MHz,CD3OD) δ: 197.7 (C-4),168.7 (C-7),165.5 (C-5),164.9 (C-9),159.0 (C-4′),131.4 (C-1′),129.0 (C-2′,6′),116.3 (C-3′,5′),103.3 (C-10),97.2 (C-6),96.3 (C-8),80.5 (C-2),44.0 (C-3)。以上数据与文献报道一致[8],故鉴定化合物9为柚皮素。
化合物10:黄色针状结晶(甲醇)。ESI-MS m/z: 255 [M-H]-,257 [M+H]+;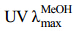 (nm): 240,336。1H-NMR (500 MHz,CD3OD) δ: 7.93 (1H,d,J = 8.9 Hz,H-6′),7.76 (1H,d,J = 15.4 Hz,H-β),7.58 (2H,d,J = 8.6 Hz,H-2,6),7.57 (1H,d,J = 15.4 Hz,H-α),6.84 (2H,d,J = 8.6 Hz,H-3,5),6.41 (1H,dd,J = 8.9,2.4 Hz,H-5′),6.29 (1H,d,J = 2.4 Hz,H-3′);13C-NMR (125 MHz,CD3OD) δ: 193.5 (C=O),167.4 (C-4′),166.3 (C-2′),161.5 (C-4),145.6 (C-β),133.3 (C-6′),131.8 (C-2,6),127.8 (C-1),118.3 (C-α),116.9 (C-3,5),114.7 (C-1′),109.1 (C-5′),103.8 (C-3′)。以上数据与文献报道一致[9],故鉴定化合物10为异甘草素。
(nm): 240,336。1H-NMR (500 MHz,CD3OD) δ: 7.93 (1H,d,J = 8.9 Hz,H-6′),7.76 (1H,d,J = 15.4 Hz,H-β),7.58 (2H,d,J = 8.6 Hz,H-2,6),7.57 (1H,d,J = 15.4 Hz,H-α),6.84 (2H,d,J = 8.6 Hz,H-3,5),6.41 (1H,dd,J = 8.9,2.4 Hz,H-5′),6.29 (1H,d,J = 2.4 Hz,H-3′);13C-NMR (125 MHz,CD3OD) δ: 193.5 (C=O),167.4 (C-4′),166.3 (C-2′),161.5 (C-4),145.6 (C-β),133.3 (C-6′),131.8 (C-2,6),127.8 (C-1),118.3 (C-α),116.9 (C-3,5),114.7 (C-1′),109.1 (C-5′),103.8 (C-3′)。以上数据与文献报道一致[9],故鉴定化合物10为异甘草素。
化合物11:淡黄色晶体(甲醇)。ESI-MS m/z: 475 [M+HCOO]-,431 [M+H]+;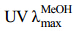 (nm): 238,266,304。1H-NMR (500 MHz,CD3OD) δ: 8.25 (1H,s,H-2),8.16 (1H,d,J = 8.9 Hz,H-5),7.49 (2H,d,J = 8.9 Hz,H-2′,6′),7.26 (1H,d,J = 2.3 Hz,H-8),7.23 (1H,dd,J = 8.9,2.3 Hz,H-6),7.00 (2H,d,J = 8.9 Hz,H-3′,5′),5.12 (1H,d,J = 7.5 Hz,H-1″),3.83 (3H,s,4′-OCH3);13C-NMR (125 MHz,CD3OD) δ: 178.0 (C-4),163.5 (C-7),161.2 (C-4′),159.3 (C-8a),155.3 (C-2),131.4 (C-2′,6′),128.3 (C-5),126.0 (C-1′),125.3 (C-3),120.2 (C-4a),117.1 (C-6),114.9 (C-3′,5′),104.9 (C-8),55.7 (4′-OCH3);glucose: 101.8 (C-1″),74.7 (C-2″),77.8 (C-3″),71.2 (C-4″),78.4 (C-5″),62.4 (C-6″)。以上数据与文献报道一致[10],故鉴定化合物11为芒柄花苷。
(nm): 238,266,304。1H-NMR (500 MHz,CD3OD) δ: 8.25 (1H,s,H-2),8.16 (1H,d,J = 8.9 Hz,H-5),7.49 (2H,d,J = 8.9 Hz,H-2′,6′),7.26 (1H,d,J = 2.3 Hz,H-8),7.23 (1H,dd,J = 8.9,2.3 Hz,H-6),7.00 (2H,d,J = 8.9 Hz,H-3′,5′),5.12 (1H,d,J = 7.5 Hz,H-1″),3.83 (3H,s,4′-OCH3);13C-NMR (125 MHz,CD3OD) δ: 178.0 (C-4),163.5 (C-7),161.2 (C-4′),159.3 (C-8a),155.3 (C-2),131.4 (C-2′,6′),128.3 (C-5),126.0 (C-1′),125.3 (C-3),120.2 (C-4a),117.1 (C-6),114.9 (C-3′,5′),104.9 (C-8),55.7 (4′-OCH3);glucose: 101.8 (C-1″),74.7 (C-2″),77.8 (C-3″),71.2 (C-4″),78.4 (C-5″),62.4 (C-6″)。以上数据与文献报道一致[10],故鉴定化合物11为芒柄花苷。
化合物12:白色固体。ESI-MS m/z: 417 [M-H]-,835 [2M-H]-,419 [M+H]+,837 [2M+H]+;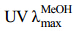 (nm): 238,276,312。1H-NMR (500 MHz,CD3OD) δ: 7.73 (1H,d,J = 8.7 Hz,H-5),7.44 (2H,d,J = 8.7 Hz,H-2′,6′),7.15 (2H,d,J = 8.7 Hz,H-3′,5′),6.50 (1H,dd,J = 8.7,2.3 Hz,H-6),6.37 (1H,d,J = 2.2 Hz,H-8),5.45 (1H,dd,J = 12.9,2.9 Hz,H-2),4.95 (1H,d,J = 1.59 Hz,H-1″),3.90 (1H,dd,J = 12.1,2.1 Hz,H-6″a),3.70 (1H,dd,J = 12.1,5.6 Hz,H-6″b),3.04 (1H,dd,J = 16.9,12.9 Hz,H-3a),2.73 (1H,dd,J = 16.9,3.0 Hz,H-3b);13C-NMR (125 MHz,CD3OD) δ: 193.1 (C-4),166.8 (C-7),165.3 (C-8a),159.2 (C-4′),134.4 (C-1′),129.8 (C-5),128.7 (C-2′,6′),117.7 (C-3′,5′),114.9 (C-4a),111.8 (C-6),103.8 (C-8),80.6 (C-2),44.9 (C-3);glucose: 102.1 (C-1″),74.8 (C-2″),78.1 (C-3″),71.3 (C-4″),77.9 (C-5″),62.4 (C-6″)。该化合物的氢谱和碳谱数据与化合物8非常相似,仅比化合物8多了1组葡萄糖信号,且其核磁数据与文献报道一致[11, 12],故鉴定化合物12为甘草苷。
(nm): 238,276,312。1H-NMR (500 MHz,CD3OD) δ: 7.73 (1H,d,J = 8.7 Hz,H-5),7.44 (2H,d,J = 8.7 Hz,H-2′,6′),7.15 (2H,d,J = 8.7 Hz,H-3′,5′),6.50 (1H,dd,J = 8.7,2.3 Hz,H-6),6.37 (1H,d,J = 2.2 Hz,H-8),5.45 (1H,dd,J = 12.9,2.9 Hz,H-2),4.95 (1H,d,J = 1.59 Hz,H-1″),3.90 (1H,dd,J = 12.1,2.1 Hz,H-6″a),3.70 (1H,dd,J = 12.1,5.6 Hz,H-6″b),3.04 (1H,dd,J = 16.9,12.9 Hz,H-3a),2.73 (1H,dd,J = 16.9,3.0 Hz,H-3b);13C-NMR (125 MHz,CD3OD) δ: 193.1 (C-4),166.8 (C-7),165.3 (C-8a),159.2 (C-4′),134.4 (C-1′),129.8 (C-5),128.7 (C-2′,6′),117.7 (C-3′,5′),114.9 (C-4a),111.8 (C-6),103.8 (C-8),80.6 (C-2),44.9 (C-3);glucose: 102.1 (C-1″),74.8 (C-2″),78.1 (C-3″),71.3 (C-4″),77.9 (C-5″),62.4 (C-6″)。该化合物的氢谱和碳谱数据与化合物8非常相似,仅比化合物8多了1组葡萄糖信号,且其核磁数据与文献报道一致[11, 12],故鉴定化合物12为甘草苷。
化合物13:黄色粉末。ESI-MS m/z: 577 [M-H]-,579 [M+H]+;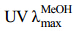 (nm): 240,278,340;1H-NMR (500 MHz,DMSO-d6) δ: 7.87 (2H,d,J = 8.3 Hz,H-2′,6′),6.93 (2H,d,J = 8.7 Hz,H-3′,5′),6.73 (1H,s,H-3),5.15 (1H,brs,H-1′′′),4.60 (1H,d,J = 9.8 Hz,H-1″),1.23 (3H,d,J = 5.8 Hz,Rha-CH3);13C-NMR (125 MHz,DMSO-d6) δ: 181.6 (C-4,C=O),165.5 (C-2),162.7 (C-7),161.2 (C-8a),160.1 (C-4′),153.8 (C-5),128.3 (C-2′,6′),121.5 (C-1′),116.1 (C-3′,5′),109.4 (C-6),103.5 (C-8),102.5 (C-4a),101.9 (C-3);glucose: 73.4 (C-1″),70.8 (C-2″),79.2 (C-3″),70.1 (C-4″),81.6 (C-5″),61.5 (C-6″);rhamnose: 77.3 (C-1′′′),74.9 (C-2′′′),74.7 (C-3′′′),72.3 (C-4′′′),72.1 (C-5′′′),18.3 (C-6′′′)。以上数据与文献报道一致[13],故鉴定化合物13为异佛来心苷。
(nm): 240,278,340;1H-NMR (500 MHz,DMSO-d6) δ: 7.87 (2H,d,J = 8.3 Hz,H-2′,6′),6.93 (2H,d,J = 8.7 Hz,H-3′,5′),6.73 (1H,s,H-3),5.15 (1H,brs,H-1′′′),4.60 (1H,d,J = 9.8 Hz,H-1″),1.23 (3H,d,J = 5.8 Hz,Rha-CH3);13C-NMR (125 MHz,DMSO-d6) δ: 181.6 (C-4,C=O),165.5 (C-2),162.7 (C-7),161.2 (C-8a),160.1 (C-4′),153.8 (C-5),128.3 (C-2′,6′),121.5 (C-1′),116.1 (C-3′,5′),109.4 (C-6),103.5 (C-8),102.5 (C-4a),101.9 (C-3);glucose: 73.4 (C-1″),70.8 (C-2″),79.2 (C-3″),70.1 (C-4″),81.6 (C-5″),61.5 (C-6″);rhamnose: 77.3 (C-1′′′),74.9 (C-2′′′),74.7 (C-3′′′),72.3 (C-4′′′),72.1 (C-5′′′),18.3 (C-6′′′)。以上数据与文献报道一致[13],故鉴定化合物13为异佛来心苷。
化合物14:黄色粉末。ESI-MS m/z: 549 [M-H]-,1 099 [2M-H]-,551 [M+H]+,1 101 [2M+H]+;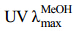 (nm): 238,278,314。1H-NMR (500 MHz,DMSO-d6),δ: 7.64 (1H,d,J = 8.7 Hz,H-5),7.45 (2H,d,J = 8.7 Hz,H-2′,6′),7.04 (2H,d,J = 8.7 Hz,H-3′,5′),6.50 (1H,dd,J = 8.7,2.2 Hz,H-6),6.35 (1H,d,J = 2.1 Hz,H-8),5.51 (1H,dd,J = 12.9,2.7 Hz,H-2),5.35 (1H,d,J = 0.8 Hz,H-1′′′),4.96 (1H,d,J = 7.5 Hz,H-1″),3.15 (1H,overlap,H-3),2.66 (1H,dd,J = 16.7,2.8 Hz,H-3);13C-NMR (125 MHz,DMSO-d6) δ: 190.1 (C-4,C=O),164.9 (C-7),163.2 (C-8a),157.4 (C-4′),132.4 (C-1′),128.5 (C-5),128.2 (C-2′,6′),116.1 (C-3′,5′),113.5 (C-4a),110.7 (C-6),102.7 (C-8),79.4 (C-2),43.2 (C-3);glucose: 98.6 (C-1″),77.1 (C-2″),77.0 (C-3″),70.0 (C-4″),75.8 (C-5″),60.7 (C-6″);apiose: 108.8 (C-1′′′),76.1 (C-2′′′),78.8 (C-3′′′),74.1 (C-4′′′),64.4 (C-5′′′)。该化合物的氢谱和碳谱数据与化合物12非常相似,仅比化合物12多了1组芹糖信号,且其核磁数据与文献报道一致[14],故鉴定化合物14为芹糖甘草苷。
(nm): 238,278,314。1H-NMR (500 MHz,DMSO-d6),δ: 7.64 (1H,d,J = 8.7 Hz,H-5),7.45 (2H,d,J = 8.7 Hz,H-2′,6′),7.04 (2H,d,J = 8.7 Hz,H-3′,5′),6.50 (1H,dd,J = 8.7,2.2 Hz,H-6),6.35 (1H,d,J = 2.1 Hz,H-8),5.51 (1H,dd,J = 12.9,2.7 Hz,H-2),5.35 (1H,d,J = 0.8 Hz,H-1′′′),4.96 (1H,d,J = 7.5 Hz,H-1″),3.15 (1H,overlap,H-3),2.66 (1H,dd,J = 16.7,2.8 Hz,H-3);13C-NMR (125 MHz,DMSO-d6) δ: 190.1 (C-4,C=O),164.9 (C-7),163.2 (C-8a),157.4 (C-4′),132.4 (C-1′),128.5 (C-5),128.2 (C-2′,6′),116.1 (C-3′,5′),113.5 (C-4a),110.7 (C-6),102.7 (C-8),79.4 (C-2),43.2 (C-3);glucose: 98.6 (C-1″),77.1 (C-2″),77.0 (C-3″),70.0 (C-4″),75.8 (C-5″),60.7 (C-6″);apiose: 108.8 (C-1′′′),76.1 (C-2′′′),78.8 (C-3′′′),74.1 (C-4′′′),64.4 (C-5′′′)。该化合物的氢谱和碳谱数据与化合物12非常相似,仅比化合物12多了1组芹糖信号,且其核磁数据与文献报道一致[14],故鉴定化合物14为芹糖甘草苷。
| [1] | Shibano M, Nukui H, Kita S, et al. Studies on index compounds for HPLC analysis of Glycyrrhiza macedonica [J]. Nat Med, 1999, 53(4): 166-172. |
| [2] | Zheng Y F, Qi L W, Cui X B, et al. Oleanane-type triterpene glucuronides from the roots of Glycyrrhiza uralensis Fischer [J]. Planta Med, 2010, 76(13): 1457-1463. |
| [3] | Shim S B, Kim N J, Kim D H. Beta-glucuronidase inhibitory activity and hepatoprotective effect of 18 beta-glycyrrhetinic acid from the rhizomes of Glycyrrhiza uralensis [J]. Planta Med, 2000, 66(1): 40-43. |
| [4] | Zapesochnaya G, Zvonkova E, Kurkin V, et al. Some properties of glycyrrhizic acid [J]. Chem Nat Compd, 1994, 30(6): 720-726. |
| [5] | Kitagawa I, Zhou J L, Sakagami M, et al. Licorice-saponins F3, G2, H2, J2, and K2, five new oleanene-triterpene oligoglycosides from the root of Glycyrrhiza uralensis [J]. Chem Pharm Bull, 1991, 39(1): 244-246. |
| [6] | Hui Z, Sheng W S, Wei L, et al. A new oleanane-type triterpene glycoside from Glycyrrhiza uralensis [J]. World Sci Technol, 2009, 11(2): 253-256. |
| [7] | Ma C J, Li G S, Zhang D L, et al. One step isolation and purification of liquiritigenin and isoliquiritigenin from Glycyrrhiza uralensis Risch. using high-speed counter-current chromatography [J]. J Chromatogr A, 2005, 1078(1/2): 188-192. |
| [8] | Fatope M O, Al-Burtomani S K S, Ochei J O, et al. Muscanone: a 3-O-(1", 8", 14"-trimethylhexadecanyl) naringenin from Commiphora wightii [J]. Phytochemistry, 2003, 62(8): 1251-1255. |
| [9] | Zhao X B, Mei W L, Gong M F, et al. Antibacterial activity of the flavonoids from Dalbergia odorifera on Ralstonia solanacearum [J]. Molecules, 2011, 16(12): 9775-9782. |
| [10] | Yu D H, Bao Y M, Wei C L, et al. Studies of chemical constituents and their antioxidant activities from Astragalus mongholicus Bunge [J]. Biomed Environ Sci, 2005, 18(5): 297-301. |
| [11] | 刘勤, 刘永隆. 黄甘草化学成分的研究 [J]. 药学学报, 1989, 24(7): 525-531. |
| [12] | Lyu H N, Kwak H Y, Lee D Y, et al. Isolation of flavonoids from processed Aconiti tuber [J]. J Appl Biol Chem, 2008, 51(4): 165-168. |
| [13] | Flamini G. Flavonoids and other compounds from the aerial parts of Viola etrusca [J]. Chem Biodivers, 2007, 4(2): 139-144. |
| [14] | Montoro P, Maldini M, Russo M, et al. Metabolic profiling of roots of liquorice (Glycyrrhiza glabra) from different geographical areas by ESI/MS/MS and determination of major metabolites by LC-ESI/MS and LC-ESI/MS/MS [J]. J Pharm Biomed Anal, 2011, 54(3): 535-544. |
 2015, Vol. 46
2015, Vol. 46


