2. 齐齐哈尔医学院, 黑龙江 齐齐哈尔 161042
2. Qiqihar Medical College, Qiqihar 161042, China
臭椿Ailanthus altissima (Mill.) Swingle为苦木科(Simaroubaceae)臭椿属Ailanthus Desf. 落叶乔木,又名山椿、樗树、白椿等,是中国本土树种,除少数省区外,全国各地均有分布。臭椿皮也称樗白皮,臭椿根皮也称椿根皮,具有清热燥湿、收涩固肠、抗肿瘤、抗菌、抗病毒等作用,是我国民间常用的中草药[1]。此外,臭椿根部表现出极强的化感活性,根部周围土壤提取物能够明显抑制多种植物种子发芽和生根,树皮及根皮提取物具有较强的杀虫活性,也可做为除虫剂[2]。为进一步开发利用臭椿植物资源,在前期对臭椿树枝化学成分研究[3]的基础上,对臭椿树根甲醇浸出液正己烷萃取物的化学成分进行了研究,得到14个化合物,分别鉴定为20β-羟基达玛烷-24-烯-3-酮(20-hydroxydammar- 24-en-3-one,1)、β-谷甾醇(β-sitosterol,2 )、ocotillone(3)、5,6,7,8-四甲氧基香豆素(5,6,7,8-tetramethoxy- coumarin,4)、尼洛替星(niloticin,5)、isofouquierone peroxide(6)、20S,24S-二羟基达玛烷-25-烯-3-酮(20S,24S-dihydroxydammar-25-en-3-one,7)、匹西狄醇A(piscidinol A,8)、eichlerianic acid(9)、二氢尼洛替星(10)、12β,20β-二羟基达玛烷-24-烯-3-酮(dammar-24-ene-12β,20β-diol-3-one,11)、铁屎 米-6-酮(canthin-6-one,12)、12β-hydroxy ocotillone (13)、20S,25-epoxy-24R-hydroxy-3-dammaranone(14)。其中,化合物3、4、6、9、10、13系首次从该植物中分离得到。
1 仪器与材料
X-6显微熔点测定仪(北京泰克仪器有限公司);Bruker AM-400和Bruker-600 MHz型核磁共振仪(德国Bruker公司);AUTOPOL V型旋光仪(美国鲁道夫公司);高效液相色谱仪:HITACHI L-7100泵,HITACHI L-3350示差折光检测器,GL SCIRNCES Inc. Inertsil PREP-ODS(250 mm×10 mm,5 μm)不锈钢柱;柱色谱用硅胶为青岛海洋化工厂产品(200~300目);薄层色谱硅胶板为烟台化工厂生产,实验用有机溶剂均为分析纯。
实验材料2012年5月3日采于辽宁省凌源市,经齐齐哈尔大学植物学教授沙伟鉴定为臭椿Ailanthus altissima (Mill.) Swingle的根,标本(Ams-201200503)保存于齐齐哈尔大学天然产物研究室。
2 提取与分离
干燥臭椿树根10 kg切碎,每次用35 L甲醇室温浸泡3 d后滤过,重复5次,合并浸出液,减压浓缩至2.5 L,加水1.9 L,依次用正己烷、醋酸乙酯、正丁醇萃取,浓缩萃取液得到正己烷萃取物84.1 g、醋酸乙酯萃取物88.7 g、正丁醇萃取物68.3 g。
取正己烷萃取物(59.0 g)用硅胶柱色谱分离,依次用正己烷-醋酸乙酯(9∶1、1∶1、0∶1)洗脱,TLC监测合并相同流分,得到10个部分F1~F10。
F6(16.7 g)用硅胶柱色谱分离,依次用正己烷-醋酸乙酯(90∶10、85∶15、80∶20、60∶40、1∶1)洗脱得到6个部分F6-1~6-6。F6-4(8.7 g)用硅胶柱色谱分离,依次用正己烷-醋酸乙酯(8∶2)、醋酸乙酯洗脱,得化合物1(416.1 mg)、3(433.0 mg);F6-5(3.8 g)用硅胶柱色谱分离,依次用正己烷-醋酸乙酯(8∶2、1∶1)洗脱得化合物2(2.0 g)和其他5个部分F6-5-1~6-5-5;F6-5-4(650.1 mg)用半制备HPLC(正己烷-醋酸乙酯8∶2,体积流量4 mL/min)分离,得到化合物4(7.9 mg,tR=13.0 min)、5(127.1 mg,tR=14.8 min);F6-5-4(529.9 mg)用半制备HPLC(正己烷-醋酸乙酯9∶1,体积流量4 mL/min)分离,得化合物6(9.0 mg,tR=28.2 min)、7(6.4 mg,tR=32.2 min)、8(8.4 mg,tR=36.7 min)。F7(15.6 g)经硅胶柱色谱分离,正己烷-醋酸乙酯(8∶2、7∶3)洗脱得化合物9(450.2 mg)和其他9个部分F7-1~7-9,F7-7(260.1 mg)用半制备HPLC(正己烷-醋酸乙酯9∶1,体积流量4 mL/min)分离,得化合物10(22.7 mg,tR=25.4 min);F7-8(4.6 g)用硅胶柱色谱分离,依次用正己烷-醋酸乙酯(8∶2、7∶3)洗脱,得化合物11(2.6 g)。F8(2.1 g)用醋酸乙酯重结晶得化合物12(70.0 mg);母液用硅胶柱色谱分离,正己烷-醋酸乙酯(8.5∶1.5、6∶4)洗脱,得到6个部分(F8-1~8-6)。F8-5(280.1 mg)用半制备HPLC(正己烷-醋酸乙酯8∶2,体积流量4 mL/min)分离,得化合物1 3(130.0 mg,tR=25.3 min)。F9(2.8 g)用硅胶柱色谱分离,正己烷-醋酸乙酯(1∶1、0∶1)洗脱,得到5个部分F9-1~9-5。F9-4(409.8 mg)用半制备HPLC(正己烷-醋酸乙酯7∶3,体积流量4 mL/min)分离,得化合物14(21.9 mg,tR=28.0 min)。
3 结构鉴定
化合物1:白色针晶(醋酸乙酯),mp 171~172 ℃;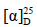 +10° (c 0.5, MeOH)。1H-NMR (600 MHz, CDCl3) δ: 5.12 (1H, t, J = 7.2 Hz, H-23), 2.49 (1H, m, H-2), 2.43 (1H, m, H-2), 2.05 (2H, m, H-23), 1.92 (1H, m, H-1), 1.85 (1H, m, H-1), 1.75 (2H, t, J = 7.5 Hz, H-16), 1.69 (3H, s, H-26), 1.67 (1H, m, H-5), 1.63 (3H, s, H-27), 1.60~1.20 (15H, m, H-6, 7, 9, 11, 12, 13, 15, 17, 22), 1.15 (3H, s, H-21), 1.08 (3H, s, H-18), 1.04 (3H, s, H-28), 1.00 (3H, s, H-29), 0.94 (3H, s, H-19), 0.89 (3H, s, H-30);13C-NMR (150 MHz, CDCl3) δ: 218.1 (C-3), 131.6 (C-25), 124.7 (C-24), 75.3 (C-20), 55.3 (C-5), 50.2 (C-14), 50.0 (C-9), 49.8 (C-17), 47.4 (C-4), 42.4 (C-13), 40.4 (C-22), 40.3 (C-8), 39.9 (C-1), 36.8 (C-10), 34.5 (C-7), 34.1 (C-2), 31.1 (C-15), 27.5 (C-28), 26.7 (C-16), 25.7 (C-26), 25.5 (C-12), 24.8 (C-21), 22.5 (C-23), 22.0 (C-29), 21.0 (C-11), 19.6 (C-6), 17.7 (C-27), 16.3 (C-30), 16. 0 (C-18), 15.2 (C-19)。以上数据与文献报道基本一致[4],故鉴定化合物1为20β-羟基-达玛烷-24-烯-3-酮。
+10° (c 0.5, MeOH)。1H-NMR (600 MHz, CDCl3) δ: 5.12 (1H, t, J = 7.2 Hz, H-23), 2.49 (1H, m, H-2), 2.43 (1H, m, H-2), 2.05 (2H, m, H-23), 1.92 (1H, m, H-1), 1.85 (1H, m, H-1), 1.75 (2H, t, J = 7.5 Hz, H-16), 1.69 (3H, s, H-26), 1.67 (1H, m, H-5), 1.63 (3H, s, H-27), 1.60~1.20 (15H, m, H-6, 7, 9, 11, 12, 13, 15, 17, 22), 1.15 (3H, s, H-21), 1.08 (3H, s, H-18), 1.04 (3H, s, H-28), 1.00 (3H, s, H-29), 0.94 (3H, s, H-19), 0.89 (3H, s, H-30);13C-NMR (150 MHz, CDCl3) δ: 218.1 (C-3), 131.6 (C-25), 124.7 (C-24), 75.3 (C-20), 55.3 (C-5), 50.2 (C-14), 50.0 (C-9), 49.8 (C-17), 47.4 (C-4), 42.4 (C-13), 40.4 (C-22), 40.3 (C-8), 39.9 (C-1), 36.8 (C-10), 34.5 (C-7), 34.1 (C-2), 31.1 (C-15), 27.5 (C-28), 26.7 (C-16), 25.7 (C-26), 25.5 (C-12), 24.8 (C-21), 22.5 (C-23), 22.0 (C-29), 21.0 (C-11), 19.6 (C-6), 17.7 (C-27), 16.3 (C-30), 16. 0 (C-18), 15.2 (C-19)。以上数据与文献报道基本一致[4],故鉴定化合物1为20β-羟基-达玛烷-24-烯-3-酮。
化合物2:无色针晶(醋酸乙酯),mp 135.1~136.2 ℃。1H-NMR (600 MHz, CDCl3) δ: 5.33 (1H, dd, J = 5.0, 2.0 Hz, H-6), 3.51 (1H, tt, J = 8.5, 4.5 Hz, H-3), 1.00 (3H, s, H-19), 0.92 (3H, d, J = 6.8 Hz, H-21), 0.83 (3H, t, J = 7.3 Hz, H-26), 0.82 (3H, d, J = 6.8 Hz, H-28), 0.80 (3H, d, J = 6.8 Hz, H-29), 0.67 (3H, s, H-18)。以上数据与文献报道基本一致[5],故鉴定化合物2为β-谷甾醇。
化合物3:无色羽状晶体(氯仿),mp 186~187.5 ℃;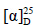 −94° (c 0.5, MeOH)。1H-NMR (600 MHz, CDCl3) δ: 3.73 (1H, t, J = 7.2 Hz, H-24), 2.49 (1H, m, H-2), 2.43 (1H, m, H-2), 1.21 (3H, s, H-21), 1.14 (3H, s, H-26), 1.12 (3H, s, H-27), 1.08 (3H, s, H-19), 1.04 (3H, s, H-28), 0.99 (3H, s, H-29), 0.93 (3H, s, H-30), 0.88 (3H, s, H-18);13C-NMR (150 MHz, CDCl3) δ: 218.0 (C-3), 86.3 (C-20), 83.3 (C-24), 71.4 (C-25), 55.3 (C-5), 50.1 (C-14), 50.0 (C-9), 49.5 (C-17), 47.4 (C-4), 43.8 (C-13), 40.3 (C-8), 39.9 (C-1), 36.9 (C-10), 35.7 (C-22), 34.6 (C-7), 34.1 (C-2), 31.4 (C-15), 27.5 (C-26), 27.4 (C-16), 26.7 (C-29), 26.1 (C-23), 25.7 (C-12), 24.3 (C-27), 23.6 (C-21), 22.1 (C-11), 21.0 (C-28), 19.7 (C-6), 16.4 (C-30), 16.0 (C-18), 15.1 (C-19)。以上数据与文献报道基本一致[4],故鉴定化合物3为ocotillone。
−94° (c 0.5, MeOH)。1H-NMR (600 MHz, CDCl3) δ: 3.73 (1H, t, J = 7.2 Hz, H-24), 2.49 (1H, m, H-2), 2.43 (1H, m, H-2), 1.21 (3H, s, H-21), 1.14 (3H, s, H-26), 1.12 (3H, s, H-27), 1.08 (3H, s, H-19), 1.04 (3H, s, H-28), 0.99 (3H, s, H-29), 0.93 (3H, s, H-30), 0.88 (3H, s, H-18);13C-NMR (150 MHz, CDCl3) δ: 218.0 (C-3), 86.3 (C-20), 83.3 (C-24), 71.4 (C-25), 55.3 (C-5), 50.1 (C-14), 50.0 (C-9), 49.5 (C-17), 47.4 (C-4), 43.8 (C-13), 40.3 (C-8), 39.9 (C-1), 36.9 (C-10), 35.7 (C-22), 34.6 (C-7), 34.1 (C-2), 31.4 (C-15), 27.5 (C-26), 27.4 (C-16), 26.7 (C-29), 26.1 (C-23), 25.7 (C-12), 24.3 (C-27), 23.6 (C-21), 22.1 (C-11), 21.0 (C-28), 19.7 (C-6), 16.4 (C-30), 16.0 (C-18), 15.1 (C-19)。以上数据与文献报道基本一致[4],故鉴定化合物3为ocotillone。
化合物4:浅黄色无定形物(醋酸乙酯),mp 54~56 ℃。1H-NMR (600 MHz, CDCl3) δ: 7.94 (1H, d, J = 9.6 Hz, H-4), 6.29 (1H, d, J = 9.6 Hz, H-3), 4.04 (3H, s, 5-OCH3), 3.98 (3H, s, 7-OCH3), 3.97 (3H, s, 6-OCH3), 3.90 (3H, s, 8-OCH3);13C-NMR (150 MHz, CDCl3) δ: 160.4 (C-2), 150.7 (C-7), 145.1 (C-6), 144.3 (C-5), 142.4 (C-8), 138.8 (C-4), 136.9 (C-9), 114.1 (C-3), 109.5 (C-10), 62.1 (5-OCH3), 61.9 (7-OCH3), 61.8 (6-OCH3), 61.5 (8-OCH3)。以上数据与文献报道基本一致[6],故鉴定化合物4为5,6,7,8-四甲氧基香豆素。
化合物5:无色针晶(醋酸乙酯),mp 146.2~147.6 ℃。1H-NMR (600 MHz, CDCl3) δ: 5.32 (1H, brd, J = 3.0 Hz, H-7), 3.58 (1H, dt, J = 8.2, 6.4 Hz, H-23), 2.74 (1H, dt, J = 14.5, 5.6 Hz, H-2a), 2.64 (1H, d, J = 8.2 Hz, H-24), 2.27 (1H, m, H-2b), 2.23 (1H, dt, J = 14.0, 3.3 Hz, H-6a), 2.07 (2H, m, H-6b, 9), 2.03 (1H, m, H-1a), 1.98 (1H, m, H-1b), 1.80 (1H, m, H-5), 1.34 (3H, s, H-26), 1.32 (3H, s, H-27), 1.12 (3H, s, H-19), 1.05 (3H, s, H-28), 1.02 (3H, s, H-30), 1.01 (3H, s, H-29), 0.96 (1H, d, J = 6.1 Hz, H-21), 0.81 (3H, s, H-18);13C-NMR (150 MHz, CDCl3) δ: 216.9 (C-3), 145.7 (C-8), 118.0 (C-7), 69.3 (C-23), 68.4 (C-24), 60.3 (C-25), 53.3 (C-17), 52.4 (C-5), 51.2 (C-14), 48.5 (C-9), 47.9 (C-4), 43.6 (C-13), 40.7 (C-22), 38.6 (C-1), 35.0 (C-10), 34.9 (C-2), 34.0 (C-15), 33.6 (C-12), 33.5 (C-20), 28.8 (C-16), 27.4 (C-30), 24.9 (C-28), 24.6 (C-27), 24.4 (C-6), 21.8 (C-29), 21.6 (C-26), 19.9 (C-21), 19.8 (C-19), 18.3 (C-11), 12.8 (C-18)。以上数据与文献报道基本一致[7],故鉴定化合物5为尼洛替星& lt; /span>。
化合物6:无色针晶(醋酸乙酯),mp 80~81 ℃;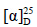 +37° (c 0.3, CHCl3)。1H-NMR (600 MHz, CDCl3) δ: 5.79 (1H, dt, J = 15.8, 7.4 Hz, H-23), 5.62 (1H, d, J = 15.8 Hz, H-24), 2.53 (1H, m, H-2a), 2.43 (1H, m, H-2b), 2.20 (2H, m, H-22), 1.92 (1H, m, H-1), 1.86 (1H, m, H-1), 1.74 (2H, m, H-5, 16a), 1.68 (1H, m, H-16b), 1.60~1.40 (7H, m, H-6, 7, 12, 13, 17), 1.38 (1H, m, H-15), 1.36 (3H, s, H-26), 1.35 (3H, s, H-27), 1.35~1.20 (4H, m, H-9, 11, 15), 1.14 (3H, s, H-21), 1.11 (1H, m, H-12), 1.08 (3H, s, H-30), 1.04 (1H, m, H-29), 1.00 (3H, s, H-28), 0.94 (3H, s, H-19), 0.88 (3H, s, H-18);13C-NMR (150 MHz, CDCl3) δ: 218.1 (C-3), 137.3 (C-24), 127.2 (C-23), 82.1 (C-25), 75.0 (C-20), 55.3 (C-5), 50.2 (C-14), 50.1 (C-9), 49.9 (C-17), 47.4 (C-4), 43.3 (C-13), 42.5 (C-22), 40.3 (C-8), 39.9 (C-1), 36.8 (C-10), 34.5 (C-7), 34.1 (C-2), 31.1 (C-15), 27.5 (C-27), 26.7 (C-26), 25.8 (C-12), 24.8 (C-16), 24.4 (C-28), 24.1 (C-21), 21.9 (C-11), 21.0 (C-29), 19.6 (C-6), 16.3 (C-30), 16.0 (C-18), 15.2 (C-19)。以上数据文献报道一致[8],故鉴定化合物6为isofouquierone peroxide。
+37° (c 0.3, CHCl3)。1H-NMR (600 MHz, CDCl3) δ: 5.79 (1H, dt, J = 15.8, 7.4 Hz, H-23), 5.62 (1H, d, J = 15.8 Hz, H-24), 2.53 (1H, m, H-2a), 2.43 (1H, m, H-2b), 2.20 (2H, m, H-22), 1.92 (1H, m, H-1), 1.86 (1H, m, H-1), 1.74 (2H, m, H-5, 16a), 1.68 (1H, m, H-16b), 1.60~1.40 (7H, m, H-6, 7, 12, 13, 17), 1.38 (1H, m, H-15), 1.36 (3H, s, H-26), 1.35 (3H, s, H-27), 1.35~1.20 (4H, m, H-9, 11, 15), 1.14 (3H, s, H-21), 1.11 (1H, m, H-12), 1.08 (3H, s, H-30), 1.04 (1H, m, H-29), 1.00 (3H, s, H-28), 0.94 (3H, s, H-19), 0.88 (3H, s, H-18);13C-NMR (150 MHz, CDCl3) δ: 218.1 (C-3), 137.3 (C-24), 127.2 (C-23), 82.1 (C-25), 75.0 (C-20), 55.3 (C-5), 50.2 (C-14), 50.1 (C-9), 49.9 (C-17), 47.4 (C-4), 43.3 (C-13), 42.5 (C-22), 40.3 (C-8), 39.9 (C-1), 36.8 (C-10), 34.5 (C-7), 34.1 (C-2), 31.1 (C-15), 27.5 (C-27), 26.7 (C-26), 25.8 (C-12), 24.8 (C-16), 24.4 (C-28), 24.1 (C-21), 21.9 (C-11), 21.0 (C-29), 19.6 (C-6), 16.3 (C-30), 16.0 (C-18), 15.2 (C-19)。以上数据文献报道一致[8],故鉴定化合物6为isofouquierone peroxide。
化合物7:无色针晶(醋酸乙酯),mp 92~93.5 ℃;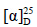 +60° (c 1.25, MeOH)。1H-NMR (600 MHz, CDCl3) δ: 5.02 (2H, m, H-26), 4.29 (1H, t, J = 6.0 Hz, H-24), 2.51 (1H, dt, J = 14.0, 7.5 Hz, H-2a), 2.43 (1H, dt, J = 14.0, 4.5 Hz, H-2b), 1.92 (1H, m, H-1), 1.82 (1H, m, H-1), 1.76 (3H, s, H-27), 1.14 (3H, s, H-21), 1.08 (3H, s, H-18), 1.04 (3H, s, H-28), 0.99 (3H, s, H-29), 0.94 (3H, s, H-19), 0.88 (3H, s, H-30);13C-NMR (150 MHz, CDCl3) δ: 218.1 (C-3), 143.6 (C-25), 114.2 (C-26), 76.4 (C-24), 75.1 (C-20), 55.7 (C-5), 50.6 (C-9), 50.5 (C-17), 50.4 (C-14), 47.6 (C-4), 42.8 (C-13), 40.6 (C-8), 40.2 (C-1), 37.1 (C-10), 37.0 (C-22), 34.8 (C-7), 34.3 (C-2), 31.4 (C-15), 29.4 (C-23), 27.7 (C-12), 26.9 (C-28), 25.1 (C-16), 25.0 (C-21), 22.3 (C-11), 21.1 (C-29), 19.9 (C-6), 17.6 (C-27), 16.5 (C-30), 16.1 (C-18), 15.3 (C-19)。以上数据与文献报道基本一致[9],故鉴定化合物7为20S,24S-二羟基达玛烷-25-烯-3-酮。
+60° (c 1.25, MeOH)。1H-NMR (600 MHz, CDCl3) δ: 5.02 (2H, m, H-26), 4.29 (1H, t, J = 6.0 Hz, H-24), 2.51 (1H, dt, J = 14.0, 7.5 Hz, H-2a), 2.43 (1H, dt, J = 14.0, 4.5 Hz, H-2b), 1.92 (1H, m, H-1), 1.82 (1H, m, H-1), 1.76 (3H, s, H-27), 1.14 (3H, s, H-21), 1.08 (3H, s, H-18), 1.04 (3H, s, H-28), 0.99 (3H, s, H-29), 0.94 (3H, s, H-19), 0.88 (3H, s, H-30);13C-NMR (150 MHz, CDCl3) δ: 218.1 (C-3), 143.6 (C-25), 114.2 (C-26), 76.4 (C-24), 75.1 (C-20), 55.7 (C-5), 50.6 (C-9), 50.5 (C-17), 50.4 (C-14), 47.6 (C-4), 42.8 (C-13), 40.6 (C-8), 40.2 (C-1), 37.1 (C-10), 37.0 (C-22), 34.8 (C-7), 34.3 (C-2), 31.4 (C-15), 29.4 (C-23), 27.7 (C-12), 26.9 (C-28), 25.1 (C-16), 25.0 (C-21), 22.3 (C-11), 21.1 (C-29), 19.9 (C-6), 17.6 (C-27), 16.5 (C-30), 16.1 (C-18), 15.3 (C-19)。以上数据与文献报道基本一致[9],故鉴定化合物7为20S,24S-二羟基达玛烷-25-烯-3-酮。
化合物8:无色针晶(丙酮),mp 179~180 ℃;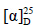 −176° (c 0.5, MeOH)。1H-NMR (400 MHz, CDCl3) δ: 5.31 (1H, t, J = 4.5 Hz, H-7), 4.12 (1H, dd, J = 4.4, 8.8 Hz, H-23), 3.17 (1H, s, H-24), 1.32 (3H, s, H-26), 1.31 (3H, s, H-27), 1.12 (3H, s, H-19), 1.05 (3H, s, H-29), 1.02 (3H, s, H-28), 1.01 (3H, s, H-30), 0.94 (3H, d, J = 6.4 Hz, H-21), 0.82 (3H, s, H-18);13C-NMR (100 MHz, CDCl3) δ: 217.1 (C-3), 145.7 (C-8), 117.9 (C-7), 75.1 (C-24), 74.3 (C-25), 69.7 (C-23), 53.8 (C-17), 52.3 (C-5), 51.2 (C-14), 48.4 (C-9), 47.8 (C-13), 43.5 (C-4), 40.5 (C-22), 38.5 (C-1), 35.0 (C-10), 34.9 (C-2), 34.0 (C-12), 33.8 (C-15), 33.7 (C-20), 28.4 (C-16), 27.3 (C-27), 27.3 (C-30), 26.2 (C-26), 24.5 (C-28), 24.3 (C-6), 22.0 (C-18), 21.5 (C-29), 18.9 (C-21), 18.3 (C-11), 12.7 (C-19)。以上数据与文献报道基本一致[10],故鉴定化合物8为匹西狄醇A。
−176° (c 0.5, MeOH)。1H-NMR (400 MHz, CDCl3) δ: 5.31 (1H, t, J = 4.5 Hz, H-7), 4.12 (1H, dd, J = 4.4, 8.8 Hz, H-23), 3.17 (1H, s, H-24), 1.32 (3H, s, H-26), 1.31 (3H, s, H-27), 1.12 (3H, s, H-19), 1.05 (3H, s, H-29), 1.02 (3H, s, H-28), 1.01 (3H, s, H-30), 0.94 (3H, d, J = 6.4 Hz, H-21), 0.82 (3H, s, H-18);13C-NMR (100 MHz, CDCl3) δ: 217.1 (C-3), 145.7 (C-8), 117.9 (C-7), 75.1 (C-24), 74.3 (C-25), 69.7 (C-23), 53.8 (C-17), 52.3 (C-5), 51.2 (C-14), 48.4 (C-9), 47.8 (C-13), 43.5 (C-4), 40.5 (C-22), 38.5 (C-1), 35.0 (C-10), 34.9 (C-2), 34.0 (C-12), 33.8 (C-15), 33.7 (C-20), 28.4 (C-16), 27.3 (C-27), 27.3 (C-30), 26.2 (C-26), 24.5 (C-28), 24.3 (C-6), 22.0 (C-18), 21.5 (C-29), 18.9 (C-21), 18.3 (C-11), 12.7 (C-19)。以上数据与文献报道基本一致[10],故鉴定化合物8为匹西狄醇A。
化合物9:白色脂状物;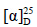 +41° (c 0.96, CHCl3)。1H-NMR (600 MHz, CDCl3) δ: 4.85 (1H, s, H-28), 4.66 (1H, s, H-28), 3.74 (1H, t, J = 7.2 Hz, H-24), 2.38 (1H, m, H-2), 2.19 (1H, m, H-2), 1.97 (1H, brd, J = 10.7 Hz, H-5), 1.73 (3H, s, H-29), 1.20 (3H, s, H-27), 1.13 (3H, s, H-26), 1.12 (3H, s, H-21), 0.99 (3H, s, H-18), 0.89 (3H, s, H-30), 0.84 (3H, s, H-19);13C-NMR (150 MHz, CDCl3) δ: 179.8 (C-3), 147.5 (C-4), 113.5 (C-28), 86.4 (C-20), 83.3 (C-24), 71.6 (C-25), 50.8 (C-17), 50.4 (C-14), 49.5 (C-9), 43.0 (C-13), 41.1 (C-5), 40.0 (C-8), 39.1 (C-10), 35.8 (C-22), 34.3 (C-2), 33.9 (C-7), 31.5 (C-6), 31.5 (C-15), 27.4 (C-27), 27.2 (C-16), 26.2 (C-23), 25.7 (C-12), 24.6 (C-1), 24.2 (C-26), 23.5 (C-21), 23.2 (C-29), 22.1 (C-11), 20.1 (C-19), 16.4 (C-30), 15.3 (C-18)。以上数据与文献报道基本一致[4],故鉴定化合物9为eichlerianic acid。
+41° (c 0.96, CHCl3)。1H-NMR (600 MHz, CDCl3) δ: 4.85 (1H, s, H-28), 4.66 (1H, s, H-28), 3.74 (1H, t, J = 7.2 Hz, H-24), 2.38 (1H, m, H-2), 2.19 (1H, m, H-2), 1.97 (1H, brd, J = 10.7 Hz, H-5), 1.73 (3H, s, H-29), 1.20 (3H, s, H-27), 1.13 (3H, s, H-26), 1.12 (3H, s, H-21), 0.99 (3H, s, H-18), 0.89 (3H, s, H-30), 0.84 (3H, s, H-19);13C-NMR (150 MHz, CDCl3) δ: 179.8 (C-3), 147.5 (C-4), 113.5 (C-28), 86.4 (C-20), 83.3 (C-24), 71.6 (C-25), 50.8 (C-17), 50.4 (C-14), 49.5 (C-9), 43.0 (C-13), 41.1 (C-5), 40.0 (C-8), 39.1 (C-10), 35.8 (C-22), 34.3 (C-2), 33.9 (C-7), 31.5 (C-6), 31.5 (C-15), 27.4 (C-27), 27.2 (C-16), 26.2 (C-23), 25.7 (C-12), 24.6 (C-1), 24.2 (C-26), 23.5 (C-21), 23.2 (C-29), 22.1 (C-11), 20.1 (C-19), 16.4 (C-30), 15.3 (C-18)。以上数据与文献报道基本一致[4],故鉴定化合物9为eichlerianic acid。
化合物10:无色针晶(醋酸乙酯),mp 177.5~178.5 ℃。1H-NMR (600 MHz, CDCl3) δ: 5.27 (1H, dd, J = 6.4, 3.0 Hz, H-7), 3.58 (1H, dt, J = 8.2, 6.4 Hz, H-23), 3.24 (1H, dd, J = 11.6, 4.1 Hz, H-3), 2.67 (1H, d, J = 8.2 Hz, H-24), 2.19 (1H, m, H-6a), 2.14 (1H, m, H-6b), 2.03 (1H, m, H-9), 1.96 (1H, m, H-2a), 1.80 (1H, m, H-2b), 1.34 (3H, s, H-26), 1.32 (3H, s, H-27), 0.99 (3H, s, H-19), 0.98 (3H, s, H-30), 0.96 (1H, d, J = 6.1 Hz, H-21), 0.86 (3H, s, H-28), 0.82 (3H, s, H-29), 0.75 (3H, s, H-18);13C-NMR (150 MHz, CDCl3) δ: 145.6 (C-8), 118.1 (C-7), 79.3 (C-3), 69.3 (C-23), 68.5 (C-24), 60.3 (C-25), 53.3 (C-17), 51.2 (C-14), 50.6 (C-5), 48.9 (C-9), 43.6 (C-13), 40.7 (C-22), 39.0 (C-4), 37.2 (C-1), 34.9 (C-10), 34.0 (C-12), 33.6 (C-15), 33.6 (C-20), 28.8 (C-2), 27.7 (C-16), 27.6 (C-28), 27.2 (C-30), 24.9 (C-27), 24.0 (C-6), 21.8 (C-18), 20.0 (C-21), 19.9 (C-26), 18.2 (C-11), 14.7 (C-29), 13.1 (C-19)。以上数据与文献报道一致[10],故鉴定化合物10为二氢尼洛替星。
化合物11:白色针晶(醋酸乙酯),mp 188.9~191.0 ℃;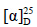 +80° (c 0.5, MeOH)。1H-NMR (600 MHz, CDCl3) δ: 5.17 (1H, t, J = 7.2 Hz, H-24), 3.61 (1H, ddd, J = 10.4, 10.4, 5.2 Hz, H-12), 2.51 (1H, m, H-2), 2.43 (1H, m, H-2), 2.17 (1H, m, H-1), 2.05 (2H, m, H-22), 1.97 (1H, m, H-1), 1.85 (2H, m, H-11), 1.75 (1H, t, J = 10.6 Hz, H-5), 1.70 (3H, s, H-26), 1.64 (3H, s, H-27), 1.60~1.23 (13H, m, H-6, 7, 9, 13, 15, 16, 17, 22), 1.20 (3H, s, H-21), 1.08 (3H, s, H-18), 1.04 (3H, s, H-28), 1.02 (3H, s, H-29), 0.98 (3H, s, H-19), 0.89 (3H, s, H-30);13C-NMR (150 MHz, CDCl3) δ: 217.8 (C-3), 132.0 (C-25), 124.8 (C-24), 74.6 (C-20), 70.6 (C-12), 55.3 (C-5), 53.3 (C-17), 51.6 (C-14), 49.3 (C-9), 48.0 (C-13), 47.4 (C-4), 39.7 (C-1), 39.7 (C-8), 36.8 (C-10), 34.1 (C-2), 34.2 (C-7), 34.3 (C-22), 31.5 (C-15), 30.9 (C-11), 27.0 (C-28), 26.7 (C-21), 26.4 (C-16), 25.7 (C-26), 22.3 (C-23), 21.0 (C-29), 19.6 (C-6), 17.7 (C-27), 16.7 (C-30), 15.9 (C-18), 15.4 (C-19)。以上数据与文献报道基本一致[3],故鉴定化合物11为12β,20β-二羟基-达玛烷-24-烯- 3-酮。
+80° (c 0.5, MeOH)。1H-NMR (600 MHz, CDCl3) δ: 5.17 (1H, t, J = 7.2 Hz, H-24), 3.61 (1H, ddd, J = 10.4, 10.4, 5.2 Hz, H-12), 2.51 (1H, m, H-2), 2.43 (1H, m, H-2), 2.17 (1H, m, H-1), 2.05 (2H, m, H-22), 1.97 (1H, m, H-1), 1.85 (2H, m, H-11), 1.75 (1H, t, J = 10.6 Hz, H-5), 1.70 (3H, s, H-26), 1.64 (3H, s, H-27), 1.60~1.23 (13H, m, H-6, 7, 9, 13, 15, 16, 17, 22), 1.20 (3H, s, H-21), 1.08 (3H, s, H-18), 1.04 (3H, s, H-28), 1.02 (3H, s, H-29), 0.98 (3H, s, H-19), 0.89 (3H, s, H-30);13C-NMR (150 MHz, CDCl3) δ: 217.8 (C-3), 132.0 (C-25), 124.8 (C-24), 74.6 (C-20), 70.6 (C-12), 55.3 (C-5), 53.3 (C-17), 51.6 (C-14), 49.3 (C-9), 48.0 (C-13), 47.4 (C-4), 39.7 (C-1), 39.7 (C-8), 36.8 (C-10), 34.1 (C-2), 34.2 (C-7), 34.3 (C-22), 31.5 (C-15), 30.9 (C-11), 27.0 (C-28), 26.7 (C-21), 26.4 (C-16), 25.7 (C-26), 22.3 (C-23), 21.0 (C-29), 19.6 (C-6), 17.7 (C-27), 16.7 (C-30), 15.9 (C-18), 15.4 (C-19)。以上数据与文献报道基本一致[3],故鉴定化合物11为12β,20β-二羟基-达玛烷-24-烯- 3-酮。
化合物12:无色针晶(醋酸乙酯),mp 156~157 ℃。1H-NMR (600 MHz, CDCl3) δ: 8.81 (1H, d, J = 5.0 Hz, H-2), 8.64 (1H, d, J = 8.2 Hz, H-8), 8.18 (1H, d, J = 8.2 Hz, H-11), 8.01 (1H, d, J = 9.7 Hz, H-4), 7.92 (1H, d, J = 5.0 Hz, H-1), 7.69 (1H, t, J = 8.2 Hz, H-9), 7.52 (1H, t, J = 8.2 Hz, H-10), 6.98 (1H, d, J = 9.7 Hz, H-5);13C-NMR (150 MHz, CDCl3) δ: 159.4 (C-6), 145.8 (C-2), 139.6 (C-4), 139.4 (C-3a), 136.2 (C-7a), 131.9 (C-11c), 130.8 (C-11b), 130.2 (C-5), 128.9 (C-11a), 125.6 (C-9), 124.3 (C-11), 122.6 (C-10), 117.2 (C-8), 116.3 (C-1)。以上数据与文献对照基本一致[3],故鉴定化合物12为铁屎米-6-酮。
化合物13:针状透明晶体(醋酸乙酯),mp 172~173 ℃。1H-NMR (600 MHz, CDCl3) δ: 3.88 (1H, dd, J = 10.8, 5.4 Hz, H-24), 3.54 (1H, m, H-12), 2.51 (1H, m, H-2), 2.44 (1H, m, H-2), 1.28 (3H, s, H-26), 1.24 (3H, s, H-27), 1.11 (3H, s, H-21), 1.08 (3H, s, H-19), 1.05 (3H, s, H-28), 1.04 (3H, s, H-29), 0.97 (3H, s, H-30), 0.92 (3H, s, H-18);13C-NMR (150 MHz, CDCl3) δ: 217.8 (C-3), 87.5 (C-24), 87.1 (C-20), 70.4 (C-12), 70.1 (C-25), 55.3 (C-5), 52.2 (C-14), 49.6 (C-9), 49.0 (C-13), 48.9 (C-17), 47.4 (C-4), 39.7 (C-1), 39.6 (C-8), 36.9 (C-10), 34.1 (C-7), 32.2 (C-2), 32.1 (C-11), 31.7 (C-15), 29.7 (C-22), 28.9 (C-27), 28.5 (C-23), 28.1 (C-21), 26.7 (C-28), 25.1 (C-16), 24.3 (C-26), 20.1 (C-29), 19.7 (C-6), 17.7 (C-18), 16.1 (C-30), 15.1 (C-19)。以上数据与文献报道基本一致[11],故鉴定化合物13为12β-hydroxy ocotillone。
化合物14:针状透明晶体(醋酸乙酯),mp 175~176 ℃。1H-NMR (600 MHz, CDCl3) δ: 3.40 (1H, brd, J = 10.2 Hz, H-24), 2.50 (1H, m, H-2), 2.43 (1H, m, H-2), 1.92 (1H, m, H-1), 1.82 (1H, m, H-12), 1.77 (1H, m, H-17), 1.73 (1H, m, H-22), 1.67~1.25 (18H, m, H-1, 5, 6, 7, 9, 11, 12, 13, 15, 16, 22, 23), 1.23 (3H, s, H-27), 1.18 (3H, s, H-26), 1.16 (3H, s, H-21), 1.08 (3H, s, H-28), 1.04 (3H, s H-29), 1.00 (3H, s, H-18), 0.95 (3H, s, H-18), 0.89 (3H, s H-30);13C-NMR (150 MHz, CDCl3) δ: 218.1 (C-3), 78.8 (C-24), 75.5 (C-20), 73.1 (C-25), 55.3 (C-5), 50.3 (C-14), 49.8 (C-17), 48.9 (C-9), 47.4 (C-4), 42.6 (C-13), 40.2 (C-8), 39.8 (C-1), 37.1 (C-22), 36.8 (C-10), 34.5 (C-7), 34.1 (C-2), 31.1 (C-15), 27.4 (C-12), 26.7 (C-28), 26.5 (C-27), 25.6 (C-16), 25.2 (C-21), 24.9 (C-23), 23.3 (C-26), 22.0 (C-29), 20.9 (C-11), 19.6 (C-6), 16.3 (C-30), 16.0 (C-19), 15.2 (C-18)。以上数据与文献报道一致[3],故鉴定化合物14为20S,25-epoxy-24R-hydroxy-3-dammaranone。
| [1] | 江苏新医学院编. 中药大辞典 [M]. 上海: 上海科学技术出版社, 1985. |
| [2] | Lu J, Wu S. Bioactivity of essential oil from Ailanthus altissima bark against 4 major stored-grain insects [J]. Afr J Microbiol Res, 2010, 4(3): 154-157. |
| [3] | 刘继梅, 张中伟, 姚佳, 等. 臭椿树枝化学成分研究 [J]. 林产化学与工业, 2013, 33(4): 121-127. |
| [4] | Aalbersberg W, Singh Y. Dammarane triterpenoids from Dysoxylum richll [J]. Phytochemistry, 1991, 30(3): 921-926. |
| [5] | 徐菁, 高鸿悦, 马淑丽, 等. 马兰化学成分及生物活性研究 [J]. 中草药, 2014, 45(22): 3246-3250. |
| [6] | Yang P, Douglas K A. Coumarin constituents of the Chinese tallow tree (Sapium sebiferum) [J]. J Nat Prod, 1985, 48(3): 486-488. |
| [7] | Alexander I G, Prabha B, Peter G. New Protolimonoids rrom the Phellodendron chinensis [J]. Phytochemistry, 1988, 27(6): 1805-1808. |
| [8] | Lee I S, Oh S R, Ahn K S, et al. Semialactone, isofouquierone peroxide and fouquierone, three new dammarane triterpenes from Rhus javanica [J]. Chem Pharm Bull, 2001, 49(8): 1024-1026. |
| [9] | Malinovskaya G V, Novikov V L, Denisenko V A, et al. A new triterpene from the leaves of Betula mandschurica [J]. Chem Nat Compd, 1980, 16(3): 257-261. |
| [10] | Hideji I, Etsuko K, Hiroshi M, et al. Cytotoxic quassinoids and tirucallane-type tritepenes from the woods of Eurycma longifolia [J]. Chem Pharm Bull, 1992, 40(4): 1053-1055. |
| [11] | Appendino G, Gariboldi P, Wollenweber E. et al. Triterpenes from the frond exudates of the fern Notholeana Greggii [J]. Phytochemistry, 1992, 31(3): 923-927. |
 2015, Vol. 46
2015, Vol. 46


