2. 中国医学科学院 北京协和医学院药用植物研究所, 北京 100193
2. Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100193, China
咖啡酸(caffeic acid,CA)化学名为3, 4-二羟基肉桂酸,是一种天然存在的酚酸类化合物,广泛分布于植物界中,如水果、谷物、咖啡、蔬菜等[1, 2],近年来得到了广泛的关注和研究,并逐步应用在食品、医药及化妆品等领域中。咖啡酸作为一种天然抗氧化剂,不仅可以影响多种食物的稳定性和营养价值,还具有多方面的生物活性,如心血管保护作用、抗菌、抗病毒、抗肿瘤等活性[3, 4, 5, 6]。咖啡酸的许多天然或人工合成的衍生物也具有与咖啡酸类似的药理作用。如绿原酸(chlorogenic acid)及咖啡酸苯乙酯(CAPE)等是植物中主要的木脂素成分,并能作为多酚氧化酶的底物,具有抗氧化活性。CAPE提自蜂胶,属酚类物质,为咖啡酸的天然酯类衍生物,近来研究发现其具有与咖啡酸类似甚至更强的抗炎、抗氧化、免疫调节等生物活性[7, 8]。
近年来在调节血脂代谢紊乱疾病的预防及治疗中,传统中草药的应用份额越来越重,研究学者们对中草药活性成分的研究也越来越深入。民族药露兜簕为海南当地用于治疗高脂血症且临床疗效显著的药物,本课题组在前期研究中发现,露兜簕中含有的天然产物咖啡酸同样具有较好的调血脂作用,但其作用机制、构效关系的深入阐述仍不明确。鉴于天然产物结构改造是开发新药的重要途径,且目前咖啡酸酯类衍生物的调脂活性仍未被报道,因此本实验设计合成了一系列咖啡酸酯类衍生物C1~10,其中化合物C5为未见文献报道的咖啡酸类新化合物;并采用HepG2为测试细胞株首次对这些衍生物进行体外抗脂质代谢紊乱活性评价[9],并初步探讨其构效关系,以期为进一步发现调脂活性良好的化合物,为中药新药的研究与开发奠定实验基础。
1 仪器与试剂艾卡(IKA○R)C-MAG HS 7型磁力搅拌器,艾卡(IKA○R)RV10 basic型旋转蒸发仪,梅特勒-托利多(METTLER TOLEDO AL104)电子天平,Bruker Avance III 600型核磁共振波谱仪,赛默飞世尔(Thermofisher)LTQ-Obitrap XL液质联用仪,IR谱采用Analect RFX—65A型红外光谱仪测定(KBr压片法),BYLABUV—III紫外灯(北京炳洋科技有限公司)。柱色谱用硅胶及薄层色谱用硅胶G、H、GF254和柱色谱用硅胶(青岛海洋化工有限公司),常规试剂市售分析纯和化学纯。
人肝癌细胞(HepG2细胞)由中国医学科学院北京协和医学院药用植物研究所药理毒理研究中心提供。
2 方法 2.1 目标化合物的设计咖啡酸的极性较大,体内代谢很快。鉴于CAPE具有较好的生物活性,设计将咖啡酸的羧基与不同的醇酯化,得到一系列咖啡酸酯类衍生物。在考察咖啡酸结构中的羧基是否为其调脂活性必需基团的同时,希望得到调脂活性优于先导化合物咖啡酸的天然产物衍生物[10, 11]。
所设计目标化合物是图 1中的化合物C1~5和化合物C6~10。其中取代基R1分别为苄基(化合物C1)、苯乙基(化合物C2)、正辛基(化合物C3)、异丁基(化合物C4)、顺-3-己烯基(化合物C5);取代基R2分别正丁基(化合物C6)、甲基(化合物C7)、乙基(化合物C8)、正丙基(化合物C9)、异丙基(化合物C10)。
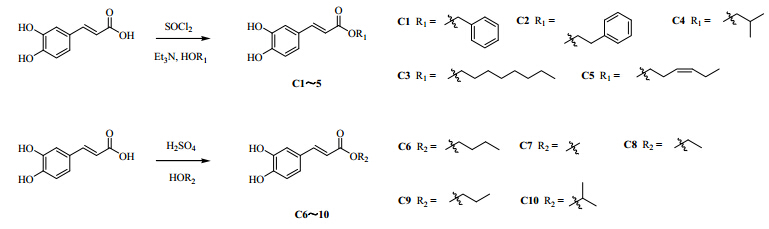 | 图 1 咖啡酸衍生物的合成路线Fig. 1 Synthetic routes of caffeic acid ester derivatives |
本实验对咖啡酸酯类衍生物的合成采用两种方法,见图 1。通过将咖啡酸的羧基酰氯化,在缚酸剂三乙胺的作用下与各种醇进行酰化反应得到化合物C1~5;通过浓硫酸做脱水剂,催化咖啡酸与各种低沸点醇进行酯化反应得到化合物C6~10。
2.3 合成方法 2.3.1 咖啡酸衍生物C1~5的合成参考文献的合成方法[11],于100 mL的圆底烧瓶中准确称取1.0 g(5.5 mmol)咖啡酸,加入30 mL二氧六环溶解。冰浴下缓慢滴加4.0 mL(55.5 mmol)的SOCl2,并于油浴中回流反应2 h。随后将各种醇55.5 mmol缓慢滴加入反应液中继续回流反应,直到TCL监测反应结束。减压蒸除溶剂,残余物通过硅胶柱色谱纯化,得到咖啡酸衍生物C1~5。
2.3.2 咖啡酸衍生物C6~10的合成参考文献的合成方法[12],于50 mL的圆底烧瓶中准确称取0.5 g(2.8 mmol)咖啡酸,加入20 mL各种脂肪醇。于冰浴下缓慢滴加0.5 mL的浓H2SO4(96%),并于油浴中回流反应,直到TCL监测反应结束。反应完全后冷却至室温,用100 mL醋酸乙酯稀释,5% NaHCO3洗至中性,有机层用无水Na2SO4干燥。减压蒸除溶剂,残余物通过硅胶柱色谱纯化,得到咖啡酸衍生物C6~10。
2.4 细胞学实验方法[12]人肝癌HepG2细胞系采用含10%胎牛血清和青霉素/链霉素(100 μg/mL)的DMEM培养基,于37 ℃、5% CO2的培养箱中培养。细胞长至培养皿70%~80%时,使用100 μmol/L油酸和棕榈酸混合液刺激细胞12 h,制成脂质堆积模型;随后给予不同的咖啡酸衍生物(10 μmol/L)以及同等浓度的咖啡酸和辛伐他汀(阳性对照),外加一个空白组对照,均孵育6 h。实验结束后使用油红O染色,通过分光分度仪在358 nm下测定吸光度(A)值,观察咖啡酸酯类化合物中具有抑制脂质堆积的有效化合物。每个实验均重复3次。
3 结果与讨论 3.1 化合物实验数据化合物C1:淡黄色粉末。收率35.2%。IR νmaxKBr(cm−1): 3 467, 3 325, 3 210, 1 689, 1 685, 1 636, 1 601, 1 278, 1 185;HR-MS m/z: 269.081 0;calcd for C16H13O4 [M-H]− 269.081 4。1H-NMR (600 MHz, CD3OD) δ: 7.58 (1H, d, J = 16.0 Hz, H-7), 7.40~7.35 (4H, m, Ph-H-2′, 3′, 5′, 6′), 7.33~7.32 (1H, m, Ph-H-4′), 7.04 (1H, d, J = 2.0 Hz, H-2), 6.95 (1H, dd, J = 2.0, 16.1 Hz, H-5), 6.77 (1H, d, J = 8.1 Hz, H-6), 6.30 (1H, d, J = 15.8 Hz, H-8), 5.21 (2H, s, H-7′);13C-NMR (150 MHz, CD3OD) δ: 169.0 (C-9), 149.6 (C-4), 147.2 (C-7), 146.8 (C-3), 137.9 (C-1′), 129.5 (Ph-C-5′, 6′), 129.2 (Ph-C-2′, 3′), 129.1 (Ph-C-4′), 127.7 (C-1), 123.0 (C-6), 116.5 (C-8), 115.2 (C-5), 115.0 (C-2), 67.2 (C-7′)。
化合物C2:淡黄色粉末,收率33.6%。IR νmaxKBr(cm−1): 3 481, 3 326, 3 309, 1 685, 1 682, 1 636, 1 602, 1 280, 1 182;HR-MS m/z: 283.096 6,calcd for C17H15O4 [M-H]− 283.097 0。1H-NMR (600 MHz, CD3OD) δ: 7.51 (1H, d, J = 16.0 Hz, H-7), 7.31~7.26 (4H, m, Ph-H-2′, 3′, 5′, 6′), 7.22~7.20 (1H, m, Ph-H-4′), 7.02 (1H, d, J = 2.0 Hz, H-2), 6.93 (1H, dd, J = 2.0, 8.2 Hz, H-5), 6.77 (1H, d, J = 8.2 Hz, H-6), 6.22 (1H, d, J = 15.4 Hz, H-8), 4.36 (2H, t, J = 7.0 Hz, H-8′), 2.99 (2H, t, J = 7.1 Hz, H-9′);13C-NMR (150 MHz, CD3OD) δ: 169.2 (C-9), 149.6 (C-4), 147.0 (C-7), 146.8 (C-3), 139.4 (C-1′), 130.0 (Ph-C-5′, 6′), 129.5 (Ph-C-2′, 3′), 127.7 (C-1), 127.5 (Ph-C-4′), 123.0 (C-6), 116.5 (C-8), 115.2 (C-5), 115.1 (C-2), 66.1 (C-8′), 36.2 (C-7′)。
化合物C3:淡黄色粉末,收率67.1%。IR νmaxKBr(cm−1): 3 490, 3 316, 2 919, 1 683, 1 685, 1 606, 1 442, 1 283, 1 178;HR-MS m/z: 291.159 0;calcd for C17H23O4 [M-H]− 291.159 6。1H-NMR (600 MHz, CD3OD) δ: 7.53 (1H, d, J = 16.1 Hz, H-7), 7.04 (1H, d, J = 2.0 Hz, H-2), 6.94 (1H, dd, J = 2.1, 8.1 Hz, H-5), 6.78 (1H, d, J = 8.1 Hz, H-6), 6.25 (1H, d, J = 16.0 Hz, H-8), 4.16 (2H, t, J = 6.7 Hz, H-8′), 1.72~1.67 (2H, m, H-7′), 1.42~1.29 (10H, m, H-2′~6′), 0.91 (3H, t, J = 7.0 Hz, H-1′);13C-NMR (150 MHz, CD3OD) δ: 169.4 (C-9), 149.6 (C-4), 146.8 (C-3), 146.7 (C-7), 127.8 (C-1), 122.9 (C-6), 116.5 (C-8), 115.3 (C-5), 115.1 (C-2), 65.6 (C-8′), 32.9 (C-7′), 30.3 (C-6′), 30.3 (C-5′), 29.9 (C-4′), 27.1 (C-3′), 23.7 (C-2′), 14.4 (C-1′)。
化合物C4:淡黄色粉末,收率64.2%。IR νmaxKBr(cm−1): 3 467, 3 083, 2 961, 1 667, 1 605, 1 442, 1 283, 1 186;HR-MS m/z: 235.096 9,calcd for C13H15O4 [M-H]− 235.097 0。1H-NMR (600 MHz, CD3OD) δ: 7.54 (1H, d, J = 16.0 Hz, H-7), 7.04 (1H, d, J = 2.0 Hz, H-2), 6.95 (1H, dd, J = 2.1, 8.1 Hz, H-5), 6.78 (1H, d, J = 8.0 Hz, H-6), 6.26 (1H, d, J = 16.0 Hz, H-8), 3.98 (2H, d, J = 6.6 Hz, H-4′), 2.02~1.95 (1H, m, H-3′), 0.98 (6H, d, J = 6.8 Hz, H-1′, 2′);13C-NMR (150 MHz, CD3OD) δ: 169.4 (C-9), 149.5 (C-4), 146.8 (C-3), 146.8 (C-7), 127.8 (C-1), 122.9 (C-6), 116.5 (C-8), 115.2 (C-5), 115.2 (C-2), 71.6 (C-4′), 29.1 (C-3′), 19.4 (C-1′, 2′)。
化合物C5:淡黄色粉末,收率66.8%。IR νmaxKBr(cm−1): 3 437, 3 198, 2 934, 1 659, 1 654, 1 306, 1 283, 1 214;HR-MS m/z: 261.112 2,calcd for C15H17O4 [M-H]− 261.112 7。1H-NMR (600 MHz, CD3OD) δ: 7.53 (1H, d, J = 16.0 Hz, H-7), 7.03 (1H, d, J = 2.0 Hz, H-2), 6.93 (1H, dd, J = 2.1, 8.0 Hz, H-5), 6.78 (1H, d, J = 8.1 Hz, H-6), 6.24 (1H, d, J = 15.9 Hz, H-8), 5.54~5.50 (1H, m, H-4′), 5.39~5.35 (1H, m, H-3′), 4.15 (2H, t, J = 6.9 Hz, H-6′), 2.46~2.42 (2H, m, H-5′), 2.12~2.07 (2H, m, H-2′), 0.98 (3H, t, J = 7.5 Hz, H-1′);13C-NMR (150 MHz, CD3OD) δ: 169.3 (C-9), 149.5 (C-4), 146.8 (C-7), 146.8 (C-3), 135.4 (C-3′), 127.7 (C-1), 125.1 (C-4′), 122.9 (C-6), 116.5 (C-8), 115.2 (C-5), 115.1 (C-2), 73.7 (C-6′), 65.0 (C-5′), 27.8 (C-2′), 14.6 (C-1′)。
化合物C6:淡黄色粉末,收率79.0%。IR νmaxKBr(cm−1): 3 485, 3 342, 2 955, 1 683, 1 603, 1 279, 1 184;HR-MS m/z: 235.096 6,calcd for C13H15O4 [M-H]− 235.097 0。1H-NMR (600 MHz, CD3OD) δ: 7.52 (1H, d, J = 16.0 Hz, H-7), 7.03 (1H, s, H-2), 6.93 (1H, m, H-5), 6.77 (1H, m, H-6), 6.24 (1H, d, J = 16.0 Hz, H-8), 4.16 (2H, m, H-4′), 1.67 (2H, m, H-3′), 1.43 (2H, m, H-2′), 0.97 (3H, m, H-1′);13C-NMR (150 MHz, CD3OD) δ: 169.4 (C-9), 149.5 (C-4), 146.8 (C-3), 146.7 (C-7), 127.7 (C-1), 122.9 (C-6), 116.5 (C-8), 115.2 (C-5), 115.1 (C-2), 65.3 (C-4′), 31.9 (C-3′), 20.2 (C-2′), 14.0 (C-1′)。
化合物C7:淡黄色粉末。收率90.8%。IR νmaxKBr(cm−1): 3 492, 3 309, 2 956, 1 681, 1 603, 1 278, 1 178;HR-MS m/z: 193.050 2,calcd for C10H9O4 [M-H]− 193.050 1。1H-NMR (600 MHz, CD3OD) δ: 7.53 (1H, d, J = 15.9 Hz, H-7), 7.03 (1H, m, H-2), 6.92 (1H, d, J = 7.7 Hz, H-5), 6.78 (1H, d, J = 7.8 Hz, H-6), 6.24 (1H, d, J = 15.8 Hz, H-8), 3.73 (3H, s, H-1′);13C-NMR (150 MHz, CD3OD) δ: 169.7 (C-9), 149.5 (C-4), 146.9 (C-7), 146.7 (C-3), 127.7 (C-1), 122.9 (C-6), 116.5 (C-8), 115.2 (C-5), 114.8 (C-2), 52.0 (C-1′)。
化合物C8:淡黄色粉末,收率89.6%。IR νmaxKBr(cm−1): 3 454, 1 661, 1 605, 1 443, 1 283;HR-MS m/z: 267.065 7,calcd for C11H11O4 [M-H]− 207.065 7。1H-NMR (600 MHz, CD3OD) δ: 7.52 (1H, d, J = 15.7 Hz, H-7), 7.04 (1H, d, J = 1.6 Hz, H-2), 6.92 (1H, dd, J = 1.8, 7.7 Hz, H-5), 6.79 (1H, d, J = 8.2 Hz, H-6), 6.23 (1H, d, J = 15.9 Hz, H-8), 4.19 (2H, q, J = 7.2 Hz, H-2′), 1.28 (3H, t, J = 7.2 Hz, H-1′);13C-NMR (150 MHz, CD3OD) δ: 169.3 (C-9), 149.3 (C-4), 146.6 (C-7), 146.6 (C-3), 127.7 (C-1), 122.8 (C-6), 116.5 (C-8), 115.3 (C-5), 115.1 (C-2), 61.4 (C-2′), 14.5 (C-1′)。
化合物C9:淡黄色粉末。收率95.0%。IR νmaxKBr(cm−1): 3 461, 2 969, 1 665, 1 605, 1 443, 1 280, 1 185;HR-MS m/z: 221.081 2,calcd for C12H13O4 [M-H]− 221.081 4。1H-NMR (600 MHz, CD3OD) δ: 7.52 (1H, d, J = 15.9 Hz, H-7), 7.04 (1H, d, J = 2.0 Hz, H-2), 6.92 (1H, dd, J = 2.0, 8.2 Hz, H-5), 6.78 (1H, d, J = 8.1 Hz, H-6), 6.23 (1H, d, J = 15.8 Hz, H-8), 4.08 (2H, t, J = 6.8 Hz, H-3′), 1.70~1.64 (2H, m, H-2′), 0.95 (3H, t, J = 7.4 Hz, H-1′);13C-NMR (150 MHz, CD3OD) δ: 169.3 (C-9), 149.3 (C-4), 146.6 (C-7), 146.6 (C-3), 127.7 (C-1), 122.8 (C-6), 116.4 (C-8), 115.2 (C-5), 115.1 (C-2), 67.0 (C-3′), 23.0 (C-2′), 10.7 (C-1′)。
化合物C10:淡黄色粉末,收率71.5%。IR νmaxKBr(cm−1): 3 467, 3 313, 2 934, 1 680, 1 601, 1 278, 1 189;HR-MS m/z: 221.081 3,calcd for C12H13O4 [M-H]− 221.081 4。1H-NMR (600 MHz, CD3OD) δ: 7.51 (1H, d, J = 16.0 Hz, H-7), 7.04 (1H, d, J = 1.8 Hz, H-2), 6.91 (1H, dd, J = 2.0, 8.3 Hz, H-5), 6.78 (1H, d, J = 8.3 Hz, H-6), 6.21 (1H, d, J = 16.0 Hz, H-8), 5.06~5.02 (1H, m, H-3′), 1.26~1.25 (6H, m, H-1′, 2′);13C-NMR (150 MHz, CD3OD) δ: 168.8 (C-9), 149.2 (C-4), 146.5 (C-3), 146.4 (C-7), 127.7 (C-1), 122.8 (C-6), 116.4 (C-8), 115.7 (C-5), 115.1 (C-2), 68.9 (C-3′), 22.1 (C-1′, 2′)。
3.2 细胞学实验结果以原型化合物咖啡酸和调脂药物辛伐他汀为阳性对照,在10 μmol/L的浓度下,通过细胞学实验考察了衍生物C1~10对HepG2细胞脂质代谢紊乱的抑制作用。具体结果见图 2。
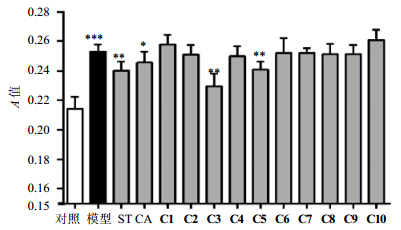 | ST-辛伐他汀 CA-咖啡酸 ST-simvastatin CA-caffeic acid 与对照组比较:*P<0.05 **P<0.01 ***P<0.001 *P < 0.05 **P < 0.01 ***P < 0.001 vs control group图 2 咖啡酸衍生物的调血脂活性筛选结果Fig. 2 Screening results for Lipid-regulating activity of caffeic acid derivatives |
药理实验结果表明,10个咖啡酸酯类衍生物均呈现出不同程度的调脂活性,其中化合物C3和C5均优于原型化合物咖啡酸和阳性对照辛伐他汀。初步的构效关系研究表明:1)咖啡酸发挥调脂活性,结构中的羧基并非为结构必需基团;2)本实验中将羧基与八个碳的脂肪醇酯化后,调脂活性最好,如化合物C3。而结构较短的脂肪烃和连有芳基的脂肪烃活性则一般,如化合物C1~2及C6~10,说明长脂肪链的引入可能有助于增加调血脂活性;3)化合物C5表现出较好的活性,且为未见文献报道的化合物,具有深入研究的价值。
| [1] | Yang S Y, Hong C O, Lee G, et al. The hepatoprotection of caffeic acid and rosmarinic acid, major compounds of erilla frutescens, against t-BHP-induced oxidative liver damage [J]. Food Chem Toxicol, 2013, 55: 92-99. |
| [2] | 刘 伟, 韩立亚, 毕艳兰. 咖啡酸衍生物的合成研究进展 [J]. 河南工业大学学报, 2014, 35(3): 98-104. |
| [3] | Lafay S, Gueux E, Rayssiguier Y, et al. Caffeic acid inhibits oxidative stress and reduces hypercholesterolemia induces by iron overload in rats [J]. Int J Vitam Nutr Res, 2005, 75(2): 119-125. |
| [4] | Cárdenas M, Marder M, Blank V C, et al. Antitumor activity of some natural flavonoids and synthetic derivatives on various human and murine cancer cell lines [J]. Bioorg Med Chem, 2006, 14(9): 2966-2971. |
| [5] | Gülçin İ. Antioxidant activity of caffeic acid (3, 4- dihydroxycinnamic acid) [J]. Toxicology, 2006, 217(2): 213-220. |
| [6] | Prasad N R, Jeyanthimala K, Ramachandran S. Caffeic acid modulates ultraviolet radiation-B induced oxidative damage in human blood lymphocytes [J]. Jhotochem Hotobiol B, 2009, 95(3): 196-203. |
| [7] | Grunberger D, Banerjee R, Eisinger K, et al. Preferential cytotoxicity on tumor cells by caffeic acid henethyl ester isolated from ropolis [J]. Experientia, 1988, 44(3): 230-232. |
| [8] | Marquez N, Sancho R, Macho A, et al. Caffeic acid henethyl ester inhibits T-cell activation by targeting both nuclear factor of activated T-cells and NF-κB transcription factors [J]. J Harmacol Exp Ther, 2004, 308(3): 993-1001. |
| [9] | Zhang X, Wu C M, Wu H F, et al. Anti-hyperlipidemic Effects and otential mechanisms of action of the caffeoylquinic acid-rich andanus tectorius fruit extract in hamsters fed a high fat-diet [J]. PLoS One, 2013, 8(4): e61922. |
| [10] | Uwai K, Osanai Y, Imaizumi T, et al. Inhibitory effect of the alkyl side chain of caffeic acid analogues on lipopolysaccharide-induced nitric oxide roduction in RAW264. 7 macrophages [J]. Bioorg Med Chem, 2008, 16(16): 7795-7803. |
| [11] | Sanderson J T, Clabault H, Patton C, et al. Antiproliferative, antiandrogenic and cytotoxic effects of novel caffeic acid derivatives in LNCaP human androgen-dependent rostate cancer cells [J]. Bioorg Med Chem, 2008, 21(22): 7182-7193. |
| [12] | Li Y, Wu C M, Liu D, et al. Chartarlactams A-P, Phenylspirodrimanes from the sponge-associated fungus stachybotrys chartarum with antihyperlipidemic activities [J]. J Nat Prod, 2014, 77(1): 138-147. |
 2014, Vol. 45
2014, Vol. 45


