2. 辽宁中医药大学药学院, 辽宁 大连 110032
2. College of Pharmacy, Liaoning University of Traditional Chinese Medicine, Dalian 110032, China
肉豆蔻Myristicae Semen是肉豆蔻科(Nutmeg)肉豆蔻属Myristica Gronov. 植物肉豆蔻Myristica fragrans Houtt. 的干燥成熟种仁,又名迦拘勒、豆蔻、肉果、顶头肉等,分布于马来西亚、印度尼西亚、西印度群岛等地,我国台湾、广东、云南等地有引种栽培。中医认为肉豆蔻具有温中行气、涩肠止泻之功效,用于脾胃虚寒、久泻不止、脘腹胀痛、食少呕吐等症。现代药理学研究表明,肉豆蔻具有抗菌、消炎、镇静、抗肿瘤等作用,但因种仁的油中富含有可致幻性的黄樟醚(safrol,质量分数>4%)、肉豆蔻醚(myristicin,质量分数>37%)等毒性物质,需经炮制后入药,其炮制作用在于“减毒增效”[1],用于治疗呕吐、腹泻、风湿病、霍乱、胃胀气等。肉豆蔻中除富含挥发油、脂肪油外,还含有苯丙素及木脂素等其他极性组分,国内外对生、制品的挥发性组分研究报道较多[2],但对中等及大极性组分、尤其是炮制前后这些成分变化与药效的相关性等研究还十分有限。杨秀伟等[3]从生品肉豆蔻中分离出多个新木脂素衍生物,也进一步说明肉豆蔻的其他药效组分还有待深入认识,这也是对其炮制原理进行深入认识的必要前提。
本课题组在前期对生品肉豆蔻进行脱脂处理后,对其中、大极性组分进行了化学成分研究,为深入研究其化学成分,本实验对生品肉豆蔻脱脂后的75%乙醇提取物进行了进一步的化学成分分离,结合理化性质及谱学分析鉴定出18个化合物,分别为香草酸(vanilic acid,1)、紫铆因(butein,2)、(2R)-3-(3′,4′,5′-三甲氧基苯基)-1,2-丙二醇[(2R)-3- (3′,4′,5′-trimethoxyphenyl)-1,2-propanediol,3]、硫磺菊素(sulphuretin,4)、3-甲氧基-4,5-亚甲二氧基肉桂酸(3-methoxy-4,5-methylenedioxy-cinnamic acid,5)、7,3′,4′-三羟基黄酮(7,3′,4′-trihydroxy- flavanone,6)、7-羟基色原酮(7-hydroxy-4- benzopyrone,7)、verrucosin(8)、(+)-赤-(7S,8R)-Δ8′- 7-羟基-3,4,3′,5′-四甲氧基-8-氧代-4′-新木脂素 [(+)-erythro-(7S,8R)-Δ8′-7-hydroxy-3,4,3′,5′- tetramethoxy-8-O-4′-neolignan,9]、(-)-赤-(7R,8S)- Δ8′-7-乙酰基-3,4,3′,5′-四甲氧基-8-氧代-4′-新木脂素 [(+)-erythro-(7R,8S)-Δ8′-7-acetoxy-3,4,3′,5′- tetramethoxy-8-O-4′-neolignan,10]、nectandrin B(11)、(-)-(7S,7′R,8S,8′R)-4,4′-二羟基-3,5,3′-三甲氧基-7,7′-环氧木脂素 [(-)-(7S,7′R,8S,8′R)-4,4′-dihydroxy-3,5,3′-trimethoxy-7,7′-epoxylignan,12]、fragransin B3(13)、fragransin B1(14)、(-)-enantiomer(15)、(-)-赤-(7R,8S)-Δ8′-7-羟基-3,4,5,3′,5′-五甲氧基-8-氧代-4′-新木脂素 [(-)-erythro- (7R,8S)-Δ8′-7-hydroxy-3,4,5,3′,5′-pentamethoxy- 8-O-4′-neolignan,16]、(+)-赤-(7S,8R)-Δ8′-7,4-二羟基-3,5,3′,5′-四甲氧基-8-氧代-4′-新木脂素 [(+)-erythro-(7S,8R)-Δ8′-7,4-dihydroxy-3,5,3′,5′- tetramethoxy-8-O-4′-neolignan,17]、(+)-5-甲氧基脱氢二异丁香酚 [(+)-5-methoxy-deydrodiisoeugenol,18]。在分离得到的化合物中,化合物8~18为木脂素类,2、4~7为从肉豆蔻属植物中首次分离得到,化合物1为从该植物中首次分离得到,结构见图 1。
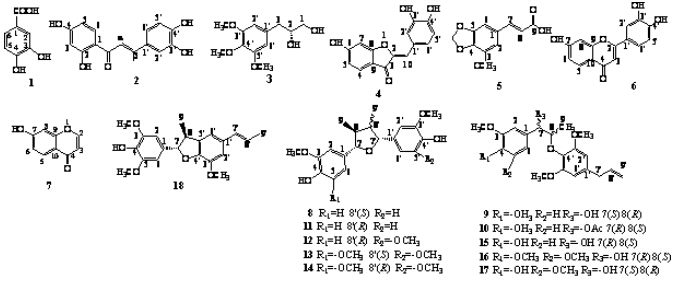 | 图 1 化合物1~18的结构Fig. 1 Structures of compounds 1—18 |
YOUNGLIN Acme 9000高效液相色谱仪;Bruker ARX—300型/AV—600型核磁共振仪(瑞士Bruker公司);分析色谱柱:RP18(250 mm×4.6 mm,5 μm);Sephadex LH-20(Pharmacia Biotech,美国);KQ—250DB型数控超声波清洗器(昆山市超声仪器有限公司);柱色谱用硅胶、薄层色谱用硅胶(青岛海洋化工有限公司);色谱甲醇、色谱乙腈(天津康科德科技有限公司);常规试剂均为分析纯(沈阳试剂厂)。
肉豆蔻于2011年购于河北安国药材市场,经沈阳药科大学袁久志副教授鉴定为肉豆蔻Myristica fragrans Houtt. 干燥成熟种仁,样品标本保存于本教研室。
2 提取与分离肉豆蔻药材10.5 kg,粉碎成小块状,先用8倍量的石油醚(沸程60~90 ℃)回流提取3次,每次2 h,以除去种油,残渣再用8倍量的体积分数为75%乙醇回流提取3次,每次2 h,减压回收溶剂得浸膏860 g。浸膏用蒸馏水分散后,依次用醋酸乙酯、正丁醇进行萃取,得醋酸乙酯提取物284 g、正丁醇提取物100 g、水层458 g。取醋酸乙酯萃取物200 g经硅胶柱色谱分离,二氯甲烷-甲醇(100∶0→0∶100)梯度洗脱,得到9个流分(Fr. 1~9)。Fr. 1(4.5 g)经过反复硅胶柱色谱法,以石油醚-醋酸乙酯为洗脱剂,经重结晶纯化,得到化合物18(8.7 mg);Fr. 2(11.2 g)经硅胶柱色谱再分离,以石油醚-醋酸乙酯为洗脱剂,得到的流分经制备型HPLC分离纯化,以甲醇-水系统为流动相进行梯度洗脱,得到化合物8(23 mg)、9(49.9 mg)、10(40.5 mg)、11(35.5 mg);Fr. 3(14.7 g)经硅胶柱色谱再分离,以二氯甲烷-甲醇为洗脱剂,得到的流分经Sephadex LH-20以甲醇为洗脱剂除掉色素,继而用制备型HPLC分离纯化,以甲醇-水为流动相进行制备分离,得到化合物12(17.8 mg)、13(12.6 mg)、14(15.7 mg)、17(10.5 mg);Fr. 4(0.8 g)经HPLC分离纯化,以甲醇-水为流动相,得到化合物7(7.8 mg)、15(12.5 mg)、16(16.6 mg);Fr. 7(10.9 g)经硅胶柱色谱再分离,以二氯甲烷-甲醇为洗脱剂,继而用制备型HPLC分离纯化(流动相为甲醇-水),制得化合物1(5.8 mg)、2(5.3 mg)、3(10.1 mg)4(6.7 mg)、5(5.4 mg)、6(8.6 mg)。
3 结构鉴定化合物1:白色结晶(甲醇),分子式C8H8O4。1H-NMR (300 MHz,DMSO-d6) δ: 3.80 (3H,s,3-OCH3),6.84 (1H,d,J = 9.0 Hz,H-6),7.42 (1H,d,J = 1.5 Hz,H-2),7.45 (1H,dd,J = 9.0,1.5 Hz,H-5)。以上数据与文献报道一致[4],故鉴定化合物1为香草酸。
化合物2:黄色粉末,分子式C15H12O5。1H-NMR (300 MHz,DMSO-d6) δ: 6.29 (1H,d,J = 1.7 Hz,H-3),6.40 (1H,dd,J = 9.0,1.7 Hz,H-5),6.82 (1H,d,J = 8.1 Hz,H-5′),7.18 (1H,d,J = 8.1 Hz,H-6′),7.26 (1H,brs,H-2′),7.60 (1H,d,J = 15.0 Hz,H-α),7.66 (1H,d,J = 15.0 Hz,H-β),8.08 (1H,d,J = 9.0 Hz,H-6);13C-NMR (150 MHz,DMSO-d6) δ: 102.6 (C-3),108.2 (C-5),113.0 (C-1),115.8 (C-5′),115.9 (C-2′),117.3 (C-α),122.5 (C-6′),126.3 (C-1′),132.9 (C-6),144.8 (C-β),145.7 (C-3′),150.0 (C-4′),165.0 (C-2),165.8 (C-4),191.5 (C=O)。以上数据与文献报道一致[5],故鉴定化合物2为紫铆因。
化合物3:白色粉末,分子式C12H18O5。HR-ESI-MS m/z: 265.109 0 [M+Na]+,[α]20D -4.0° (c 0.09,CHCl3)。1H-NMR (600 MHz,DMSO-d6) δ: 2.45 (1H,dd,J = 13.2,7.8 Hz,H-3α),2.96 (1H,dd,J = 13.2,6.0 Hz,H-3β),3.29 (2H,m,H-1),3.61 (3H,s,4′-OCH3),3.63 (1H,m,H-2),3.74 (6H,s,3′,5′-OCH3),4.53 (1H,m,1-OH),4.55 (1H,m,2-OH),6.51 (2H,brs,H-2′,6′);13C-NMR (150 MHz,DMSO-d6) δ: 40.1 (C-3),55.7 (3′,5′-OCH3),59.9 (4′-OCH3),65.4 (C-1),72.5 (C-2),106.5 (C-2′,6′),135.4 (C-1′),135.6 (C-4′),152.4 (C-3′,5′),以上数据与文献报道一致[6],故鉴定化合物3为 (2R)-3-(3′,4′,5′-三甲氧基苯基)-1,2-丙二醇。
化合物4:黄色结晶(甲醇),分子式C15H10O5。1H-NMR (600 MHz,DMSO-d6) δ: 6.63 (1H,s,H-10),6.70 (1H,brd,J = 7.4 Hz,H-5),6.74 (1H,brs,H-7),6.84 (1H,d,J = 8.0 Hz,H-4),7.24 (1H,brd,J = 8.0 Hz,H-6′),7.45 (1H,brs,H-2′),7.59 (1H,d,J = 8.3 Hz,H-5′);13C-NMR (150 MHz,DMSO-d6) δ: 98.4 (C-7),111.9 (C-10),112.9 (C-5),113.2 (C-9),116.0 (C-5′),118.0 (C-2′),123.4 (C-1′),124.5 (C-6′),125.7 (C-4),145.5 (C-2),145.6 (C-3′),148.0 (C-4′),166.2 (C-6),167.4 (C-8),181.1 (C-3)。以上数据与文献报道一致[7],故鉴定化合物4为硫磺菊素。
化合物5:白色粉末。1H-NMR (600 MHz,DMSO-d6) δ: 3.85 (3H,s,3-OCH3),6.0 (2H,s,O-CH2-O),6.42 (1H,d,J = 15.8 Hz,H-8),7.03 (2H,brs,H-2,6),7.48 (1H,d,J = 15.8 Hz,H-7),12.21 (1H,brs,-COOH);13C-NMR (150 MHz,DMSO-d6) δ: 56.4 (3-OCH3),101.4 (-OCH2-O),101.8 (C-6),109.5 (C-8),117.6 (C-2),129.1 (C-1),136.7 (C-4),143.3 (C-7),144.0 (C-5),148.9 (C-3),167.8 (C-9)。以上数据与文献报道一致[8],故鉴定化合物5为3-甲氧基- 4,5-亚甲二氧基肉桂酸。
化合物6:黄色粉末,分子式C15H10O5。1H-NMR (600 MHz,DMSO-d6) δ: 6.60 (1H,s,H-3),6.89 (3H,m,H-6,5′,6′),7.38 (1H,brs,H-8),7.39 (1H,brs,H-2′),7.85 (1H,d,J = 8.5 Hz,H-5);13C-NMR (150 MHz,DMSO-d6) δ: 102.4 (C-8),104.5 (C-3),113.2 (C-6),114.8 (C-2′),116.0 (C-10),116.1 (C-5′),118.5 (C-6′),122.1 (C-1′),126.4 (C-5),145.7 (C-3′),149.1 (C-4′),157.3 (C-9),162.5 (C-7),162.6 (C-2),176.2 (C-4)。以上数据与文献报道一致[5],故鉴定化合物6为7,3′,4′-三羟基黄酮。
化合物7:黄色粉末,分子式C9H6O2。1H-NMR (600 MHz,DMSO-d6) δ: 6.20 (1H,d,J = 6.0 Hz,H-3),6.83 (1H,d,J = 2.0 Hz,H-8),6.90 (1H,dd,J = 8.4,2.0 Hz,H-6),7.86 (1H,d,J = 8.4 Hz,H-5),8.14 (1H,d,J = 6.0 Hz,H-2),10.8 (1H,s,7-OH);13C-NMR (150 MHz,DMSO-d6) δ: 102.4 (C-8),111.9 (C-3),115.1 (C-6),117.0 (C-10),126.7 (C-5),156.1 (C-2),157.8 (C-9),162.7 (C-7),175.6 (C-4)。以上数据与文献报道一致[9],故鉴定化合物7为7-羟基色原酮。
化合物8:淡黄色油状物,香草醛浓硫酸显紫红色,分子式C20H24O5,[α]21D +30.8° (c 0.06,CHCl3)。1H-NMR (400 MHz,CDCl3) δ: 0.66 (3H,d,J = 7.0 Hz,H-9′),1.06 (3H,d,J = 6.5 Hz,H-9),1.78 (1H,m,H-8),2.24 (1H,m,H-8′),3.86 (3H,s,3′-OCH3),3.92 (3H,s,3-OCH3),4.40 (1H,d,J = 9.2 Hz,H-7),5.11 (1H,d,J = 8.4 Hz,H-7′),5.58 (1H,s,4′-OH),5.64 (1H,s,4-OH),6.82 (1H,dd,J = 8.0,1.6 Hz,H-6′),6.84 (1H,d,J = 1.6 Hz,H-2′),6.89 (1H,d,J = 8.0 Hz,H-5′),6.93 (1H,d,J = 8.0 Hz,H-5),7.00 (1H,dd,J = 8.0,1.6 Hz,H-6),7.04 (1H,d,J = 1.6 Hz,H-2);13C-NMR (100 MHz,CDCl3) δ: 15.0 (C-9,9′),46.0 (C-8′),47.7 (C-8),55.8 (3′-OCH3),55.9 (3-OCH3),83.1 (C-7′),87.3 (C-7),109.4 (C-2),109.7 (C-2′),113.9 (C-5′),114.2 (C-5),119.3 (C-6),119.9 (C-6′),132.8 (C-1′),133.2 (C-1),144.6 (C-4′),145.2 (C-4),146.2 (C-3′),146.5 (C-3)。以上数据与文献报道一致[10],故鉴定化合物8为verrucosin。
化合物9:无色油状物,分子式C22H28O6,[α]20D +4.6° (c 0.15,CHCl3)。1H-NMR (400 MHz,CDCl3) δ: 1.13 (3H,d,J = 6.6 Hz,H-9),3.37 (2H,d,J = 6.6 Hz,H-7′),3.86 (3H,s,4-OCH3),3.88 (6H,s,3′,5′-OCH3),3.89 (3H,s,3-OCH3),4.34 (1H,dq,J = 6.6,2.6 Hz,H-8),4.81 (1H,d,J = 2.6 Hz,H-7),5.98 (1H,m,H-8′),6.46 (2H,brs,H-2′,6′),5.11~5.16 (2H,m,H-9),6.76 (1H,dd,J = 8.3,1.2 Hz,H-6),6.80 (1H,d,J = 8.3 Hz,H-5),6.95 (1H,d,J = 1.2 Hz,H-2);13C-NMR (100 MHz,CDCl3) δ: 12.8 (C-9),40.6 (C-7′),56.1 (3′,5′-OCH3),55.8 (4-OCH3),55.9 (3-OCH3),72.8 (C-7),82.3 (C-8),105.4 (C-2′,6′),109.2 (C-2),110.7 (C-5),116.2 (C-9′),118.1 (C-6),132.6 (C-1),132.9 (C-1′),136.1 (C-4′),137.1 (C-8′),147.9 (C-3),148.8 (C-4),153.5 (C-3′,5′)。以上数据与文献报道一致[11],故鉴定化合物9为 (+)-赤-(7S,8R)-Δ8′-7-羟基-3,4,3′,5′-四甲氧基-8-氧代-4′-新木脂素。
化合物10:无色油状物,分子式C24H30O7,[α]20D -5.9° (c 0.46,CHCl3)。1H-NMR (400 MHz,CDCl3) δ: 1.32 (3H,d,J = 6.4 Hz,H-9),2.18 (3H,s,7-OCOCH3),3.33 (2H,d,J = 6.4 Hz,H-7′),3.78 (6H,s,3′,5′-OCH3),3.85 (3H,s,4-OCH3),3.86 (3H,s,3-OCH3),4.43 (1H,dq,J = 6.4,3.2 Hz,H-8),5.08~5.13 (2H,m,H-9′),5.86 (1H,d,J = 3.2 Hz,H-7),5.96 (1H,m,H-8′),6.39 (2H,brs,H-2′,6′),6.84 (1H,dd,J = 8.3,1.2 Hz,H-6),6.80 (1H,d,J = 8.3 Hz,H-5),6.88 (1H,d,J = 1.4 Hz,H-2);13C-NMR (100 MHz,CDCl3) δ: 14.4 (C-9),21.2 (7-OCOCH3),40.5 (C-7′),56.8 (3′,5′-OCH3),55.9 (3,4-OCH3),72.6 (C-7),80.5 (C-8),105.5 (C-2′,6′),110.2 (C-2),110.7 (C-5),115.9 (C-9′),119.3 (C-6),130.5 (C-1),133.7 (C-1′),135.7 (C-4′),137.2 (C-8′),148.5 (C-4),148.7 (C-3),153.4 (C-3′,5′),170.1 (7-OCOCH3)。以上数据与文献报道一致[11],故鉴定化合物10为 (-)-赤-(7R,8S)-Δ8′-7-乙酰氧基-3,4,3′,5′-四甲氧基-8-氧代-4′-新木脂素。
化合物11:无色油状物,分子式C20H24O5,HR-ESI-MS m/z: 367.157 3 [M+Na]+,[α]20D -10.9° (c 0.06,CH3OH)。1H-NMR (400 MHz,CDCl3) δ: 1.04 (6H,d,J = 6.6 Hz,H-9,9′),2.34 (2H,m,H-8,8′),3.88 (6H,s,3,3′-OCH3),4.51 (2H,brd,J = 6.6 Hz,H-7,7′),5.62 (2H,s,4,4′-OH),6.90 (2H,d,J = 8.0 Hz,H-5,5′),6.93 (2H,dd,J = 8.0,1.6 Hz,H-6,6′),6.97 (2H,d,J = 1.6 Hz,H-2,2′);13C-NMR (100 MHz,CDCl3) δ: 12.9 (C-9,9′),44.3 (C-8,8′),55.8 (3,3′-OCH3),87.3 (C-7,7′),109.2 (C-2,2′),114.1 (C-5,5′),119.3 (C-6,6′),134.2 (C-1,1′),145.0 (C-4,4′),146.4 (C-3,3′)。以上数据与文献报道一致[12],故鉴定化合物11为nectandrin B。
化合物12:无色油状物,分子式C21H26O6,[α]20D -4.2° (c 0.12,CH3OH)。1H-NMR (400 MHz,CDCl3) δ: 1.04 (3H,d,J = 6.8 Hz,H-9′),1.06 (3H,d,J = 6.8 Hz,H-9),2.34 (2H,m,H-8,8′),3.89 (9H,s,3,3′,5′-OCH3),4.53 (2H,t,J = 9.4 Hz,H-7,7′),6.66 (2H,brs,H-2′,6′),6.91 (1H,d,J = 8.0 Hz,H-5),6.95 (1H,dd,J = 8.0,1.6 Hz,H-6),6.97 (1H,d,J = 1.6 Hz,H-2);13C-NMR (100 MHz,CDCl3) δ: 13.0 (C-9),13.2 (C-9′),44.2 (C-8),44.6 (C-8′),56.0 (3-OCH3),56.4 (3′,5′-OCH3),87.4 (C-7′),87.7 (C-7),103.3 (C-2′,6′),109.4 (C-2),114.3 (C-5),119.4 (C-6),133.6 (C-1′),134.1 (C-1),134.2 (C-4′),145.3 (C-4),146.6 (C-3),147.1 (C-3′,5′)。以上数据与文献报道一致[13],故鉴定化合物12为(-)-(7S,7′R,8S,8′R)-4,4′-二羟基-3,5,3′-三甲氧基-7,7′-环氧木脂素。
化合物13:无色油状物,分子式C22H28O7。1H-NMR (400 MHz,CDCl3) δ: 0.68 (3H,d,J = 7.0 Hz,H-9′),1.09 (3H,d,J = 7.0 Hz,H-9),1.77 (1H,m,H-8),2.24 (1H,m,H-8′),3.87 (6H,s,3′,5′-OCH3),3.92 (6H,3,5-OCH3),4.42 (1H,d,J = 9.2 Hz,H-7),5.11 (1H,d,J = 8.6 Hz,H-7′),5.47 (1H,s,4′-OH),5.49 (1H,s,4-OH),6.57 (2H,brs,H-2′,6′),6.64 (2H,brs,H-2,6);13C-NMR (100 MHz,CDCl3) δ: 15.0 (C-9′),15.3 (C-9),46.2 (C-8′),47.9 (C-8),56.4 (3,5,3′,5′-OCH3),83.3 (C-7′),87.6 (C-7),103.0 (C-2),103.5 (C-6),103.9 (C-2′,6′),132.1 (C-1),132.5 (C-1′),133.9 (C-4′),134.5 (C-4),146.9 (C-3′,5′),147.2 (C-3,5)。以上数据与文献报道一致[13],故鉴定化合物13为fragransin B3。
化合物14:白色粉末,分子式C22H28O7,[α]20D -12.7° (c 0.06,CH3OH)。1H-NMR (400 MHz,CDCl3) δ: 1.06 (6H,d,J = 6.6 Hz,H-9,9′),2.34 (2H,m,H-8,8′),3.89 (12H,s,3,3′-OCH3),4.51 (2H,brd,J = 6.0 Hz,H-7,7′),5.49 (2H,s,4,4′-OH),6.67 (4H,d,J = 1.6 Hz,H-2,2′,6,6′);13C-NMR (100 MHz,CDCl3) δ: 13.2 (C-9,9′),44.4 (C-8,8′),56.4 (3,5,3′,5′-OCH3),87.6 (C-7,7′),103.4 (C-2,6,2′,6′),133.5 (C-1,1′),134.3 (C-4,4′),147.7 (C-3,3′,5,5′)。以上数据与文献报道一致[14],故鉴定化合物14为fragransin B1。
化合物15:无色油状物,分子式C21H26O6,[α]20D -7.7° (c 0.28,CHCl3)。1H-NMR (400 MHz,CDCl3) δ: 1.12 (3H,d,J = 6.4 Hz,H-9),3.37 (2H,d,J = 6.4 Hz,H-7′),3.88 (6H,s,3′,5′-OCH3),3.89 (3H,s,3-OCH3),4.34 (1H,dq,J = 6.4,2.7 Hz,H-8),4.79 (1H,d,J = 2.6 Hz,H-7),5.11~5.16 (2H,m,H-9′),5.55 (1H,brs,4-OH),5.99 (1H,m,H-8′),6.46 (2H,brs,H-2′,6′),6.68 (1H,dd,J = 8.3,1.2 Hz,H-6),6.84 (1H,d,J = 8.3 Hz,H-5),6.97 (1H,d,J = 1.4 Hz,H-2);13C-NMR (100 MHz,CDCl3) δ: 12.9 (C-9),40.7 (C-7′),56.1 (3-OCH3),56.3 (3′,5′-OCH3),72.9 (C-8),82.4 (C-7),105.6 (C-2′,6′),108.7 (C-2),114.0 (C-5),116.4 (C-9′),118.9 (C-6),132.2 (C-1),133.1 (C-4′),136.3 (C-1′),137.2 (C-8′),144.6 (C-4),146.6 (C-3),153.6 (C-3′,5′)。以上数据与文献报道一致[15],故鉴定化合物15为(-)-enantiomer。
化合物16:白色粉末,分子式C23H30O7,[α]20D -60.1° (c 0.06,CH3OH)。1H-NMR (400 MHz,CDCl3) δ: 1.13 (3H,d,J = 6.4 Hz,H-9),3.38 (2H,d,J = 6.7 Hz,H-7′),3.82 (3H,s,4-OCH3),3.85 (6H,s,3′,5′-OCH3),3.88 (6H,s,3,5-OCH3),4.34 (1H,m,H-8),4.79 (1H,brs,H-7),5.13 (2H,m,H-9′),5.99 (1H,m,H-8′),6.47 (2H,brs,H-2′,6′),6.54 (2H,brs,H-2,6);13C-NMR (100 MHz,CDCl3) δ: 12.9 (C-9),40.7 (C-7′),56.3 (3,5,3′,5′-OCH3),61.0 (4-OCH3),73.2 (C-8),82.3 (C-7),103.0 (C-2′,6′),105.6 (C-2,6),116.4 (C-9′),133.0 (C-4′),135.8 (C-4,1′),136.4 (C-8′),137.1 (C-1),153.2 (C-3′,5′),153.6 (C-3,5)。以上数据与文献报道一致[15],故鉴定化合物16为(-)-赤-(7R,8S)-Δ8′-7-羟基-3,4,5,3′,5′-五甲氧基-8-氧代-4′-新木脂素。
化合物17:无色油状物,分子式C22H24O7,[α]20D +9.8° (c 0.52,CHCl3)。1H-NMR (400 MHz,CDCl3) δ: 1.12 (3H,d,J = 6.4 Hz,H-9),3.37 (2H,d,J = 6.7 Hz,H-7′),3.86 (6H,s,3′,5′-OCH3),3.88 (6H,s,3,5-OCH3),4.32 (1H,m,H-8),4.78 (1H,brs,H-7),5.11~5.16 (2H,m,H-9′),5.47 (1H,brs,4-OH),5.98 (1H,m,H-8′),6.46 (2H,brs,H-2′,6′),6.54 (2H,brs,H-2,6);13C-NMR (100 MHz,CDCl3) δ: 12.9 (C-9),40.7 (C-7′),56.2 (3,5-OCH3),56.4 (3′,5′-OCH3),73.1 (C-8),82.4 (C-7),102.8 (C-2,6),105.6 (C-2′,6′),116.4 (C-9′),131.2 (C-8′),133.1 (C-1),133.6 (C-4′),136.4 (C-1′),137.1 (C-4),147.0 (C-3,5),153.6 (C-3′,5′)。以上数据与文献报道一致[16],故鉴定化合物17为 (+)-赤-(7S,8R)-Δ8′-7,4-二羟基-3,5,3′,5′-四甲氧基-8-氧代-4′-新木脂素。
化合物18:淡黄色油状物,分子式C21H24O5,[α]20D +6.7° (c 0.09,CH3OH)。1H-NMR (400 MHz,CDCl3) δ: 1.40 (3H,d,J = 6.5 Hz,H-9),1.88 (3H,m,H-9′),3.47 (1H,dq,J = 9.4,6.8 Hz,H-8),3.89 (6H,s,3,5-OCH3),3.91 (3H,s,3′-OCH3),5.08 (1H,d,J = 9.5 Hz,H-7),5.53 (1H,s,4-OH),6.12 (1H,m,H-8′),6.36 (1H,d,J = 15.5 Hz,H-7′),6.67 (2H,brs,H-2,6),6.77 (1H,brs,H-2′),6.80 (1H,brs,H-6′);13C-NMR (100 MHz,CDCl3) δ: 17.6 (C-9),18.4 (C-9′),45.7 (C-8),55.9 (3′-OCH3),56.4 (3,5-OCH3),94.1 (C-7),103.5 (C-2,6),109.2 (C-2′),113.3 (C-6′),123.6 (C-8′),130.7 (C-7′),131.2 (C-1′),132.3 (C-5′),133.2 (C-1),134.8 (C-4),144.2 (C-3′),146.5 (C-4′),147.0 (C-3,5)。以上数据与文献报道一致[17],故鉴定化合物18为(+)-5-甲氧基脱氢二异丁香酚。
| [1] | 代冬梅,贾天柱,徐洪亮,等. 肉豆蔻炮制及现代研究进展 [J]. 中成药,2005,27(12):1416-1419. |
| [2] | 王 莹,杨秀伟. 印度尼西亚产肉豆蔻挥发油成分的GC-MS分析 [J]. 中华中医药杂志,2007,22(9):603-606. |
| [3] | 杨秀伟,黄 鑫,艾合买提·买买提. 肉豆蔻中新的新木脂素类化合物 [J]. 中国中药杂志,2008,33(4):397-402. |
| [4] | 李陆军,宋 杰,冯丽彬. 变叶树参根醋酸乙酯部位化学成分研究 [J]. 现代药物与临床,2013,28(2):144-146. |
| [5] | 赵爱华,赵勤实,彭丽艳,等. 鬼针草中一个新的查耳酮甙 [J]. 云南植物研究,2004,26(1):121-126. |
| [6] | Ren X F,She X G,Peng K. First enantioselective synthesis of the neolignans rhaphidecursinol A and virolongin B [J]. J Chin Chem Soc,2004,51(5A):969-974. |
| [7] | Li Y L,Li J,Wang N L,et al,Flavonoids and a new polyacetylene from Bidens parviflora Wild. [J]. Molecules,2008,13(8):1931-1941. |
| [8] | 陈若云,于德泉. 新疆藁本有效成分研究 [J]. 药学学报,1995,30(7):526-530. |
| [9] | 李俊平,王彩芳,刘 婷,等. 河南蹄叶橐吾根的化学成分研究 [J]. 天然产物研究与开发,2011,23(6):1014-1016. |
| [10] | Enríquez R G,Chávez M A,Reynold W F. Phytochemical investigations of plants of the genus Aristolochia,1. Isolation and NMR spectral characterization of eupomatenoid derivatives [J]. J Nat Prod,1984,47(5):896-899. |
| [11] | Duan L,Tao H W,Hao X J,et al. Cytotoxic and antioxidative phenolic compounds from the traditional chinese medicinal plant,Myristica fragrans [J]. Planta Med,2009,75(11):1241-1245. |
| [12] | 史 辑,赵启苗,贾天柱. 长形肉豆蔻Myristica argentea Warb的化学成分研究 [J]. 天然产物研究与开发,2010,22(6):987-990. |
| [13] | Hattori M,Hada S,Kawata Y,et al. New 2,5-bis-aryl-3,4-dimethyltetrahydrofuran lignans from the aril of myristica fragrans [J]. Chem Pharm Bull,1987,35(8):3312-3315. |
| [14] | Pu J X,Gao X M,Lei C,et al Three new compounds from Kadsura longipedunculata [J]. Chem Pharm Bull,2008,56(8):1143-1146. |
| [15] | Kasahara H,Miyazawa M,Kameoka H,Absolute configuration of 8-O-4'-neolignans from Myristica fragrans [J]. Phytochemistry,1995,40(5):1515-1517. |
| [16] | Zacchino S A,Badano H. Synthesis of absolute configuration assignment of erythro-(3,4,5-trimethoxy-7-hydroxy-1'-allyl-2',6'-dimethoxy)-8-O-4'-neolignan [J]. J Nat Prod,1988,51(6):1261-1265. |
| [17] | Sy L K,Saunders R K,Brown G D. Phytochemistry of illiciumdunnianumi and the systematic position of the illiciaceae [J]. Phytochemistry,1997,44(6):1099-1108. |
 2014, Vol. 45
2014, Vol. 45


