2. 爱茉莉太平洋(上海)研发有限公司, 上海 201801
2. Amorepacific Shanghai R & I Center, Shanghai 201801, China
蕨麻Potentilla anserine L. 为蔷薇科(Rosaceae)委陵菜属Potentilla L. 植物鹅绒委陵菜的膨大块根,又名人参果、延寿草、蕨麻委陵菜等,藏语中称为“戳玛”、“卓老沙僧”,是藏医习用草药。蕨麻分布极广,横跨欧、亚、美洲的北半球温带,在我国主要分布于西藏、青海、新疆、云南等西南各省。蕨麻具有生津止渴、健脾益胃、益气补血、收敛止血、止泻、止咳、利痰的功效,主治吐血、下血、崩中、疟疾痈疮、脾虚腹泻、下痢等症。其富含淀粉、脂肪酸及人体所需的18种氨基酸和多种维生素,具有较高的医疗和营养价值[1]。早在清乾隆年间的《西宁府新志》中就有蕨麻的记载:“蕨麻产于野,状如麻根而色紫,食之益人,又谓延寿果”。《中药大辞典》、《西藏常用中草药》等对此药也均有记载。为了进一步更加合理地利用蕨麻这一药用资源,本实验对蕨麻块根的石油醚部位和醋酸乙酯部位的化学成分进行了系统研究,通过各种色谱技术分离得到13个化合物,经波谱方法分别鉴定为2,19α-二羟基-3-羰基-1,12-二烯-乌苏酸-28-O-β-D-葡萄糖苷(2,19α-dihydroxy-3-oxours-1,12-dien-28-oic acid 28-O-β-D-glucopyranosyl ester,1)、3β-O-乙酰坡模醇酸(3β-acetoxy-19α-hydroxyursa-12-en-28-oic acid,2)、3-表-2-氧代-坡模醇酸(3-epi-2-oxopomolic acid,3)、2-氧代-坡模醇酸(2-oxopomolic acid,4)、坡模醇酸(pomolic acid,5)、野椿酸(euscaphic acid,6)、arjunic acid(7)、蕨麻苷B(potentillanoside B,8)、2-氧代-坡模醇酸-28-O-β-D-葡萄糖苷(2- oxopomolic acid 28-O-β-D-glucopyranoside,9)、坡模醇酸-28-O-β-D-葡萄糖苷(pomolic acid-28-O-β- D-glucopyranoside,10)、刺梨苷(kajiichigoside F1,11)、野蔷薇苷(rosamultin,12)、腺嘌呤(adenine,13)。其中化合物1为新的三萜皂苷类化合物,命名为蕨麻苷G,化合物3和7为首次从该属植物中分离得到,化合物2和13为从该植物中首次分离得到。
1 仪器与材料UV759S紫外分光光度仪(上海精密科学仪器有限公司);Wzz—2s型旋光仪(上海精密科学仪器有限公司);X—5型显微熔点测定仪(北京泰克仪器公司);制备型液相色谱仪(LC—8A型泵,SPD—M10A型检测器,日本岛津制作所);INOVA—400型核磁共振波谱仪(美国Varian公司);Q-TOF micro YA019型四极杆飞行时间质谱仪(美国Waters公司);YMC-Pack ODS-AQ制备色谱柱(250 mm×20 mm,5 μm,日本YMC公司);Sephadex LH-20(GE Healthcare公司);GF254薄层色谱板和柱色谱硅胶均系烟台芝罘黄务硅胶开发试验厂生产;所用色谱纯及分析纯有机试剂均为国药集团上海化学试剂公司生产。
蕨麻于2013年6月购自青海省西宁市,经青海省藏医药研究院杜连平助理研究员鉴定为蕨麻Potentilla anserine L. 的块根,标本(20130612)保存于上海医药工业研究院中药研究部。
2 提取与分离蕨麻块根11.5 kg,粉碎后用75%乙醇于60 ℃提取4次,每次2 h,合并提取液,减压浓缩回收溶剂得浸膏3.4 kg。将浸膏加适量的水混悬,依次用石油醚(60~90 ℃)、二氯甲烷、醋酸乙酯和正丁醇萃取,减压浓缩回收溶剂,得到各相应组分。
其石油醚萃取部位(55.18 g)经硅胶柱色谱,以石油醚-醋酸乙酯梯度洗脱(100∶0→0∶100),500 mL为1流分,所得Fr. 97~107流分有晶体析出,经重结晶,得到化合物2(10.0 mg)。
醋酸乙酯萃取部位(45.10 g)经硅胶柱色谱,依次用二氯甲烷-甲醇梯度洗脱(100∶0→0∶100),TLC检测合并相同组分,共得到9个流分(Fr. 1~9)。Fr. 5(5.70 g)经硅胶柱色谱,石油醚-丙酮(9∶1→1∶1)梯度洗脱,再经Sephadex LH-20柱色谱及半制备HPLC分离纯化,得到化合物3(13.8 mg)、4(86.0 mg)、5(23.7 mg)、6(98.3 mg);Fr. 6(8.42 g)用甲醇溶解,滤过,分为2个部位,甲醇难溶部位为Fr. 6-1(4.27 g),易溶解的部位为Fr. 6-2(4.15 g);Fr. 6-2经反复硅胶柱色谱,石油醚-丙酮梯度洗脱(6∶1→1∶2)及Sephadex LH-20柱色谱、半制备型HPLC分离纯化,得到化合物1(22.1 mg)、7(10.0 mg)、8(11.2 mg);Fr. 7(2.46 g)和Fr. 8(14.90 g)经过反复硅胶柱色谱,二氯甲烷-甲醇梯度洗脱(30∶1→0∶1),再经Sephadex LH-20柱色谱及半制备HPLC分离纯化,得到化合物9(61.1 mg)、10(61.8 mg)、11(21.08 mg)、12(152.4 mg)、13(8.9 mg)。
3 结构鉴定
化合物1:白色无定形粉末(甲醇)。[α]25D +37.4° (c 2.57,MeOH);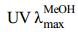 (nm): 270.0;ESI-MS m/z: 669.33 [M+Na]+,681.21 [M+Cl]-,提示相对分子质量为646。HR-ESI-MS m/z: 669.361 8 [M+Na]+(计算值为669.361 5),推断分子式为C36H54O10,不饱和度为10。1H-NMR (400 MHz,CD3OD) δ: 0.86,1.10,1.17,1.21,1.22,1.34 (各3H,s) 和0.93 (3H,d,J = 6.4 Hz,H-30);5.36 (1H,m,H-12),6.29 (1H,s,H-1) 为烯质子信号,5.32 (1H,d,J = 6.4 Hz,H-1′) 为糖端基质子信号,3.29~3.81 (6H,m) 为糖上其他质子信号。化合物1的13C-NMR (100 MHz,CD3OD) 谱显示有36个碳信号,30个为苷元上碳信号,6个为糖基碳信号。结合DEPT谱分析,δC 95.8,78.6,78.4,73.9,71.3,62.6为糖上碳信号;30个苷元碳信号中有7个亚甲基碳信号(δC 20.1,24.7,26.6,27.2,29.6,34.0,38.2),2组双键碳信号(δC 129.3,140.0)、(δC 130.3,145.9),以及δC 178.5和δC 202.5的酯羰基和酮羰基碳信号。根据以上波谱数据,提示化合物1可能为乌苏烷型三萜类化合物。由HMBC谱(图 1)可知,化合物1的糖端基质子信号H-1′ (δH 5.32) 与δC 178.5 (C-28) 相关,推断结构中的糖基与28位碳相连。
(nm): 270.0;ESI-MS m/z: 669.33 [M+Na]+,681.21 [M+Cl]-,提示相对分子质量为646。HR-ESI-MS m/z: 669.361 8 [M+Na]+(计算值为669.361 5),推断分子式为C36H54O10,不饱和度为10。1H-NMR (400 MHz,CD3OD) δ: 0.86,1.10,1.17,1.21,1.22,1.34 (各3H,s) 和0.93 (3H,d,J = 6.4 Hz,H-30);5.36 (1H,m,H-12),6.29 (1H,s,H-1) 为烯质子信号,5.32 (1H,d,J = 6.4 Hz,H-1′) 为糖端基质子信号,3.29~3.81 (6H,m) 为糖上其他质子信号。化合物1的13C-NMR (100 MHz,CD3OD) 谱显示有36个碳信号,30个为苷元上碳信号,6个为糖基碳信号。结合DEPT谱分析,δC 95.8,78.6,78.4,73.9,71.3,62.6为糖上碳信号;30个苷元碳信号中有7个亚甲基碳信号(δC 20.1,24.7,26.6,27.2,29.6,34.0,38.2),2组双键碳信号(δC 129.3,140.0)、(δC 130.3,145.9),以及δC 178.5和δC 202.5的酯羰基和酮羰基碳信号。根据以上波谱数据,提示化合物1可能为乌苏烷型三萜类化合物。由HMBC谱(图 1)可知,化合物1的糖端基质子信号H-1′ (δH 5.32) 与δC 178.5 (C-28) 相关,推断结构中的糖基与28位碳相连。
 | 图 1化合物1的结构及主要HMBC、NOESY信号相关Fig. 1 Structure and key HMBC and NOESY correlations of compound 1 |
化合物1与已知化合物kajiichigoside F1的波谱数据相似[2],只是结构中A环的波谱数据相差较大。化合物1的HMBC及HMQC谱显示,δC 202.5 (C-3) 与δH 6.29 (H-1),δC145.9 (C-2) 与δH 6.29 (H-1) 有远程相关,δH 6.29 (H-1) 与δC 130.3 (C-1) 直接相连。由以上波谱数据推断,化合物1的A环为1-双键-3-羰基六元环结构。经进一步的文献检索[3],
化合物1中苷元结构与已知化合物2,19α-dihydroxy- 3-oxours-1,12-dien-28-oic acid波谱数据基本一致,仅因为化合物1的28位碳与糖基相连,其碳谱数据较已知化合物向高场位移4.3。从糖端基信号δH 5.32 (1H,d,J = 6.4 Hz) 的耦合常数及所连糖的核磁共振13C-NMR谱数据,与文献报道比较[2],可以推出所连糖的构型为β-D-葡萄糖。此外,在NOESY谱中可以观察到H-26 (δ 0.86) 与H-29 (δ 1.21),H-27 (δ 1.34) 与H-30 (δ 0.93) 存在远程相关,因此确定19位的羟基为α型。综上所述,化合物1的结构鉴定为2,19α-二羟基-3-羰基-1,12-二烯-乌苏酸-28-O-β-D-葡萄糖苷。经过Scifinder数据库检索,确定该化合物为1个新化合物,命名为蕨麻苷G(potentillanoside G),结构见图 2。具体1H-NMR、13C-NMR谱数据归属见表 1。
 | 图 2化合物1~13的结构Fig. 2Structures of compounds 1—13 |
| 表 1 化合物1的1H-NMR和13C-NMR 数据 (400/100 MHz,CD3OD) Table 1 Alkaloids isolated from plants of Dactylicapnos Wall. |
化合物2:块状结晶(氯仿),mp 254~257 ℃。ESI-MS m/z: 537.36 [M+Na]+,513.38 [M-H]-。1H-NMR (400 MHz,CDCl3) δ: 0.74,0.86,0.88,0.96,1.21,1.26 (6×CH3,s,H-26,24,23,25,29,27),0.96 (3H,d,J = 6.4 Hz,H-30),2.04 (3H,s,CH3CO),2.54 (1H,s,H-18),5.35 (1H,t,J = 3.6 Hz,H-12);13C-NMR (100 MHz,CDCl3) δ: 38.2 (C-1),23.7 (C-2),81.0 (C-3),37.7 (C-4),55.3 (C-5),18.4 (C-6),32.7 (C-7),40.1 (C-8),47.2 (C-9),37.0 (C-10),23.6 (C-11),129.3 (C-12),138.0 (C-13),41.2 (C-14),28.3 (C-15),25.4 (C-16),47.8 (C-17),53.0 (C-18),73.1 (C-19),41.2 (C-20),26.0 (C-21),37.5 (C-22),27.5 (C-23),16.7 (C-24),15.3 (C-25),17.1 (C-26),24.5 (C-27),183.7 (C-28),28.1 (C-29),16.1 (C-30),21.2 (CH3CO),170.9 (C=O)。以上数据与文献报道一致[4],故鉴定化合物2为3β-O-乙酰坡模醇酸。
化合物3:白色无定形粉末,ESI-MS m/z: 509.38 [M+Na]+,485.31 [M-H]-。1H-NMR (400 MHz,C5D5N) δ: 0.84,0.90,0.97,1.13,1.31,1.56 (6×3H,s,H-24,25,26,23,29,27),1.00 (3H,d,J = 6.8 Hz,H-30),2.19 (2H,d,J = 12.0 Hz,H-1α),2.88 (2H,d,J = 12.0 Hz,H-1β),2.93 (1H,brs,H-18),3.80 (1H,s,H-3β),5.46 (1H,brs,H-12);13C-NMR (100 MHz,C5D5N) δ: 51.4 (C-1),213.1 (C-2),83.1 (C-3),42.3 (C-4),50.3 (C-5),19.3 (C-6),33.2 (C-7),40.8 (C-8),47.7 (C-9),42.7 (C-10),23.9 (C-11),127.5 (C-12),140.0 (C-13),42.0 (C-14),29.2 (C-15),26.4 (C-16),48.3 (C-17),54.6 (C-18),72.7 (C-19),42.3 (C-20),26.9 (C-21),38.3 (C-22),27.4 (C-23),21.7 (C-24),16.8 (C-25),17.1 (C-26),24.4 (C-27),180.5 (C-28),27.1 (C-29),16.6 (C-30)。以上数据与文献报道一致[5, 6],故鉴定化合物3为3-表-2-氧代-坡模醇酸。
化合物4:白色无定形粉末,ESI-MS m/z: 509.27 [M+Na]+,485.13 [M-H]-。1H-NMR (400 MHz,C5D5N) δ: 0.76,0.78,0.95,1.20,1.33,1.62 (6×3H,s,H-24,26,25,23,29,27),1.01 (3H,d,J = 6.8 Hz,H-30),2.94 (1H,s,H-18),4.05 (1H,s,H-3α),5.45 (1H,brs,H-12);13C-NMR (100 MHz,C5D5N) δ: 53.6 (C-1),210.9 (C-2),83.3 (C-3),45.7 (C-4),54.9 (C-5),19.1 (C-6),33.1 (C-7),40.7 (C-8),47.4 (C-9),43.6 (C-10),23.8 (C-11),127.4 (C-12),140.1 (C-13),42.2 (C-14),29.4 (C-15),26.3 (C-16),48.3 (C-17),54.6 (C-18),72.7 (C-19),42.3 (C-20),27.1 (C-21),38.3 (C-22),29.3 (C-23),17.1 (C-24),16.6 (C-25),16.7 (C-26),24.5 (C-27),180.5 (C-28),26.9 (C-29),16.3 (C-30)。与化合物3对比,化合物4的主要波谱数据差异在A环,化合物4的碳谱数据δ 53.6 (C-1),210.9 (C-2),45.7 (C-4),54.9 (C-5) 分别比化合物3对应的数据51.4 (C-1),213.1 (C-2),42.3 (C-4),50.3 (C-5) 向低场移动2.2,向高场移动了2.2,向低场移动了3.4,向低场移动了4.6,推测原因是3-OH的立体构型的变化引起了化学位移的变化,进一步与文献报道对比[7],鉴定化合物4为2-氧代-坡模醇酸。
化合物5:白色无定形粉末,ESI-MS m/z: 495.36 [M+Na]+,471.32 [M-H]-。1H-NMR (400 MHz,C5D5N) δ: 0.84,0.92,1.01,1.13,1.34,1.61 (6×3H,s,H-25,24,26,23,29,27),2.95 (1H,brs,H-18),3.33 (1H,dd,J = 9.6,5.0 Hz,H-3α),5.50 (1H,brs,H-12);13C-NMR (100 MHz,C5D5N) δ: 39.0 (C-1),28.1 (C-2),78.2 (C-3),39.3 (C-4),55.9 (C-5),18.9 (C-6),33.6 (C-7),40.4 (C-8),47.8 (C-9),37.4 (C-10),24.0 (C-11),128.1 (C-12),139.9 (C-13),42.1 (C-14),29.3 (C-15),26.4 (C-16),48.3 (C-17),54.6 (C-18),72.7 (C-19),42.3 (C-20),27.1 (C-21),38.4 (C-22),28.7 (C-23),16.7 (C-24),15.5 (C-25),17.2 (C-26),24.6 (C-27),180.5 (C-28),26.9 (C-29),16.4 (C-30)。以上数据与文献报道一致[8],故鉴定化合物5为坡模醇酸。
化合物6:白色无定形粉末,ESI-MS m/z: 511.10 [M+Na]+,487.21 [M-H]-。1H-NMR (400 MHz,DMSO-d6) δ: 0.68,0.79,0.90,0.90,1.09,1.30 (6×CH3,s,H-24,25,23,26,29,27),0.84 (3H,d,J = 6.8 Hz,H-30),2.39 (1H,s,H-18),3.17 (1H,d,J = 1.6 Hz,H-3),5.18 (1H,brs,H-12);13C-NMR (100 MHz,DMSO-d6) δ: 41.7 (C-1),64.8 (C-2),78.0 (C-3),38.1 (C-4),47.8 (C-5),21.9 (C-6),32.8 (C-7),40.3 (C-8),46.7 (C-9),37.9 (C-10),23.3 (C-11),126.9 (C-12),138.8 (C-13),41.3 (C-14),28.9 (C-15),25.3 (C-16),47.0 (C-17),53.3 (C-18),71.8 (C-19),41.5 (C-20),26.5 (C-21),37.3 (C-22),28.1 (C-23),16.2 (C-24),16.3 (C-25),17.8 (C-26),24.1 (C-27),179.0 (C-28),26.0 (C-29),16.8 (C-30)。以上数据与文献报道一致[9],故鉴定化合物6为野椿酸。
化合物7:白色无定形粉末,ESI-MS m/z: 511.38 [M+Na]+,487.39 [M-H]-。1H-NMR (400 MHz,C5D5N) δ: 0.93,0.96,0.97,1.01,1.08,1.15,1.52 (7×CH3,s,H-25,26,24,30,29,23,27),3.27 (1H,d,J = 9.6 Hz,H-3),3.51 (1H,brs,H-18),5.45 (1H,t,J = 3.6 Hz,H-12)。13C-NMR (100 MHz,C5D5N) δ: 47.5 (C-1),68.6 (C-2),83.9 (C-3),39.8 (C-4),56.1 (C-5),19.0 (C-6),33.4 (C-7),40.1 (C-8),48.3 (C-9),38.7 (C-10),24.3 (C-11),123.2 (C-12),144.9 (C-13),42.2 (C-14),29.3 (C-15),28.4 (C-16),46.1 (C-17),44.8 (C-18),81.4 (C-19),35.7 (C-20),29.3 (C-21),33.6 (C-22),29.1 (C-23),17.6 (C-24),16.8 (C-25),17.5 (C-26),24.8 (C-27),180.9 (C-28),28.7 (C-29),24.9 (C-30)。以上数据与文献报道一致[10],故鉴定化合物7为arjunic acid。
化合物8:白色无定形粉末,ESI-MS m/z: 671.33 [M+Na]+,683.29 [M+Cl]-。1H-NMR (400 MHz,C5D5N) δ: 0.85,0.95,1.06,1.12,1.27,1.53 (6×3H,s,H-24,25,26,23,29,27),0.96 (3H,d,J = 4.8 Hz,H-30),3.81 (1H,s,H-3β),5.43 (1H,brs,H-12),6.15 (1H,d,J = 8.0 Hz,H-1′);13C-NMR (100 MHz,C5D5N) δ: 51.6 (C-1),213.2 (C-2),83.1 (C-3),42.2 (C-4),50.3 (C-5),19.4 (C-6),33.1 (C-7),41.0 (C-8),47.7 (C-9),42.7 (C-10),23.9 (C-11),127.9 (C-12),139.3 (C-13),42.1 (C-14),29.2 (C-15),26.1 (C-16),48.6 (C-17),54.4 (C-18),72.7 (C-19),42.1 (C-20),26.6 (C-21),37.6 (C-22),27.4 (C-23),21.7 (C-24),16.8 (C-25),17.4 (C-26),24.3 (C-27),176.9 (C-28),26.9 (C-29),16.6 (C-30),95.8 (C-1′),74.0 (C-2′),78.9 (C-3′),71.4 (C-4′),79.1 (C-5′),62.4 (C-6′)。以上数据与文献报道一致[5],故鉴定化合物8为蕨麻苷B。
化合物9:白色无定形粉末,ESI-MS m/z: 671.23 [M+Na]+,683.18 [M+Cl]-,485.24 [M-H-162]-。1H-NMR (400 MHz,C5D5N) δ: 0.77,0.83,1.08,1.19,1.29,1.58 (6×3H,s,H-23,24,25,26,27,29),0.96 (3H,d,J = 7.2 Hz,H-30),2.82 (1H,s,H-18),5.19 (1H,s,H-3α),5.42 (1H,s,H-12),6.15 (1H,d,J = 8.0 Hz,H-1′);13C-NMR (100 MHz,C5D5N) δ: 53.7 (C-1),210.9 (C-2),83.4 (C-3),45.7 (C-4),54.9 (C-5),19.1 (C-6),33.1 (C-7),40.9 (C-8),47.4 (C-9),43.6 (C-10),23.9 (C-11),127.8 (C-12),139.4 (C-13),42.1 (C-14),29.4 (C-15),26.1 (C-16),48.6 (C-17),54.4 (C-18),72.7 (C-19),42.2 (C-20),26.6 (C-21),37.6 (C-22),29.1 (C-23),17.2 (C-24),16.6 (C-25),17.0 (C-26),24.4 (C-27),176.8 (C-28),27.0 (C-29),16.4 (C-30),95.8 (C-1′),74.0 (C-2′),78.9 (C-3′),71.4 (C-4′),79.1 (C-5′),62.5 (C-6′)。通过与化合物4对比,化合物9的δC 176.8 (C-28) 比化合物4 δC 180.5 (C-28) 向高场移动了3.7,化合物9的δH 6.15 (1H,d,J = 8.0 Hz,H-1′) 为糖端基氢信号,推测化合物9可能是化合物4的苷,进一步与文献报道[7]对比,鉴定化合物9为2-氧代-坡模醇酸-28-O-β-D-葡萄糖苷。
化合物10:白色无定形粉末,ESI-MS m/z: 657.37 [M+Na]+,669.30 [M+Cl]-。1H-NMR (400 MHz,DMSO-d6) δ: 0.69,0.69,0.86,0.90,1.09,1.28 (6×3H,s,H-25,24,26,23,29,27),0.85 (3H,d,J = 6.4 Hz,H-30),2.37 (1H,brs,H-18),5.18 (1H,brs,H-12),5.19 (1H,d,J = 8.0 Hz,H-1′);13C-NMR (100 MHz,DMSO-d6) δ: 38.4 (C-1),27.1 (C-2),77.6 (C-3),38.5 (C-4),55.0 (C-5),18.2 (C-6),32.8 (C-7),39.6 (C-8),46.9 (C-9),36.6 (C-10),23.3 (C-11),127.2 (C-12),138.2 (C-13),42.1 (C-14),28.2 (C-15),25.3 (C-16),47.5 (C-17),53.3 (C-18),71.8 (C-19),41.3 (C-20),25.9 (C-21),36.6 (C-22),28.3 (C-23),16.6 (C-24),15.2 (C-25),16.3 (C-26),23.9 (C-27),175.6 (C-28),26.5 (C-29),16.1 (C-30),94.2 (C-1′),72.4 (C-2′),76.9 (C-3′),69.8 (C-4′),77.1 (C-5′),60.9 (C-6′)。以上数据与文献报道一致[11],故鉴定化合物10为坡模醇酸-28-O-β-D-葡萄糖苷。
化合物11:白色无定形粉末,ESI-MS: m/z 673.30 [M+Na]+,685.28 [M+Cl]-。1H-NMR (400 MHz,DMSO-d6) δ: 0.66,0.78,0.89,0.89,1.11,1.28 (6×CH3,s,H-24,25,23,26,29,27),0.83 (3H,d,J = 6.0 Hz,H-30),2.38 (1H,s,H-18),3.13 (1H,d,Hβ-3),5.17 (1H,d,J = 8.4 Hz,H-1′),5.18 (1H,s,H-12);13C-NMR (100 MHz,DMSO-d6) δ: 41.9 (C-1),64.9 (C-2),78.1 (C-3),38.1 (C-4),47.8 (C-5),22.0 (C-6),32.7 (C-7),40.3 (C-8),46.7 (C-9),37.9 (C-10),23.4 (C-11),127.2 (C-12),138.3 (C-13),41.4 (C-14),28.9 (C-15),25.3 (C-16),47.5 (C-17),53.4 (C-18),71.9 (C-19),41.4 (C-20),26.6 (C-21),36.7 (C-22),28.1 (C-23),16.3 (C-24),16.3 (C-25),17.9 (C-26),24.1 (C-27),175.7 (C-28),25.9 (C-29),16.7 (C-30),94.2 (C-1′),72.5 (C-2′),77.6 (C-3′),69.8 (C-4′),76.9 (C-5′),60.9 (C-6′)。以上数据与文献报道一致[9],故鉴定化合物11为刺梨苷。
化合物12:白色无定形粉末,ESI-MS m/z: 673.28 [M+Na]+,685.30 [M+Cl]-,487.32 [M-H-162]-。1H-NMR (400 MHz,DMSO-d6) δ: 0.68,0.72,0.91,0.93,1.14,1.28 (6×3H,s,H-25,23,24,26,29,27),0.85 (3H,d,J = 6.4 Hz,H-30),2.39 (1H,s,H-18),2.75 (1H,d,J = 9.2 Hz,H-3),5.17 (1H,d,J = 8.0 Hz,H-1′),5.19 (1H,s,H-12);13C-NMR (100 MHz,DMSO-d6) δ: 46.9 (C-1),67.3 (C-2),82.5 (C-3),39.0 (C-4),55.0 (C-5),18.2 (C-6),32.7 (C-7),40.3 (C-8),47.2 (C-9),37.7 (C-10),23.4 (C-11),127.1 (C-12),138.3 (C-13),41.3 (C-14),28.1 (C-15),25.2 (C-16),47.5 (C-17),53.3 (C-18),71.8 (C-19),41.3 (C-20),25.9 (C-21),36.6 (C-22),28.9 (C-23),16.3 (C-24),16.4 (C-25),17.2 (C-26),23.9 (C-27),175.6 (C-28),26.5 (C-29),16.6 (C-30),94.2 (C-1′),72.4 (C-2′),77.6 (C-3′),69.8 (C-4′),76.9 (C-5′),60.9 (C-6′)。以上数据与文献报道一致[9],故鉴定化合物12为野蔷薇苷。
化合物13:白色粉末,ESI-MS m/z: 135 [M-H]-。1H-NMR (400 MHz,DMSO-d6) δ: 8.11 (1H,s,H-2),8.07 (1H,s,H-8),7.00 (2H,s,6-NH2);13C-NMR (100 MHz,DMSO-d6) δ: 152.5 (C-2),151.7 (C-4),117.4 (C-5),155.3 (C-6),139.7 (C-8)。以上数据与文献报道一致[12],故鉴定化合物13为腺嘌呤。
致谢:韩国爱茉莉太平洋株式会社对本实验提供的资助。| [1] | 王晋, 张坚, 康胜利, 等. 青海产蕨麻营养成分的研究 [J]. 青海医药杂志, 1998, 28(2): 52-53. |
| [2] | Liu D L, Zhu S, Ma R, et al. Triterpenoids from the roots of Rose odorata var. gigantean [J]. Chin J Nat Med, 2010, 8(1): 12-15. |
| [3] | Hattori M, Kuo K P, Shu Y Z, et al. A triterpene from the fruits of Rubus Chingii [J]. Phytochemistry, 1988, 27(12): 3975-3976. |
| [4] | Fraga B M, Díaz C E, Quintana N. Triterpenes from natural and transformed roots of Plocama pendula [J]. J Nat Prod, 2006, 69(7): 1092-1094. |
| [5] | Morikawa T, Ninomiya K, Imura K, et al. Hepatoprotective triterpenes from traditional Tibetan medicine Potentilla anserine [J]. Phytochemistry, 2014, 102: 169-181. |
| [6] | D'Abrosca B, Fiorentino A, Monaco P, et al. Radical-scavenging activities of new hydroxylated ursane triterpenes from cv. Annurca apples [J]. Chem Biodivers, 2005, 2: 953-958. |
| [7] | Jia Z J, Liu X Q, Liu Z M. Triterpenoids from Sanguisorba alpina [J]. Phytochemistry, 1993, 32(1): 155-159. |
| [8] | Amimoto K, Yoshikawa K, Arihara S. Triterpenoid saponins of aquifoliaceous plants. VIII. Ilexosides XXIX-XXXII from the leaves of Ilex rotunda Thunb. [J]. Chem Pharm Bull, 1992, 40(12): 3138-3142. |
| [9] | 褚良, 王立波, 张哲. 藏药蕨麻的化学成分研究 [J]. 中国现代中药, 2008, 10(3): 10-12. |
| [10] | Huang P, Karagianis G, Wei S X, et al. Triterpene acids from Toddalia asiatica [J]. Nat Prod Res Dev, 2005, 17(4): 404-408. |
| [11] | Sun W, Zhang Z L, Liu X, et al. Terpene glycosides from the roots of Sanguisorba officinalis L. and their hemostatic activities [J]. Molecules, 2012, 17: 7629-7636. |
| [12] | 罗舟, 苏明智, 颜鸣, 等. 蒙古黄芪的化学成分研究 [J]. 中草药, 2012, 43(3): 458-462. |
 2014, Vol. 45
2014, Vol. 45


