2. 沈阳药科大学中药学院, 辽宁 沈阳 110016;
3. 华北制药集团先泰药业有限公司 质量保证中心, 河北 石家庄 052165
2. College of Traditional Chinese Materia Medica, Shenyang Pharmaceutical University, Shenyang 110016, China;
3. Quality Assurance Center, North China Pharmaceutical Group Semisyntech Co., Ltd., Shijiazhuang 052165, China
酸枣仁为鼠李科枣属植物酸枣Ziziphus jujuba Mill. var. spinosa (Bunge) Hu ex H. F. Chou的干燥成熟种子。其药用始载于《神农本草经》,列为上品,《本草纲目》列为本部类,是养心安神首选药品[1]。其性平,味甘、酸,归肝、胆、心经,用于治疗虚烦不眠、惊悸多梦、体虚多汗、津伤口渴等症。目前,关于酸枣仁的化学成分报道主要为皂苷类、三萜酸类、黄酮类及生物碱类[2, 3, 4, 5, 6, 7, 8, 9, 10, 11]。本实验对酸枣仁的70%乙醇提取物进行化学成分研究,分离得到5个黄酮碳苷类化合物,分别鉴定为6″,6′′′-二阿魏酰异斯皮诺素(6″,6′′′-diferuloylisospinosin,1),山柰酚-3-O-β-D-吡喃木糖基-(1→2)-[α-L-吡喃鼠李糖基-(1→6)]-β-D-
吡喃葡萄糖苷(kaempferol-3-O-β-D-xylopyranosyl- (1→2)-[α-L-rhamnopyranosyl-(1→6)]-β-D-glucopyrano-side,2),斯皮诺素(spinosin,3),异斯皮诺素(isospinosin,4),6′′′-阿魏酰斯皮诺素(6′′′- feruloylspinosin,5)。化合物1为1个新的黄酮碳苷化合物,命名为6″,6′′′-二阿魏酰异斯皮诺素。化合物2为首次从该属植物中分离得到。
1 仪器与材料LCQ Advantage MAX质谱仪(美国Finnigan公司),Synapt G2质谱仪(美国Waters公司),Avance—300/400超导核磁共振仪(瑞士Bruker公司),SHIMADZU LC—6AD系列制备型高效液相色谱。制备高效液相色谱柱为C18柱(250 mm×20 mm,5 μm,Cosmosil)。薄层色谱硅胶GF254和柱色谱硅胶(青岛海洋化工厂),D-101大孔树脂(天津),ODS(日本YMC公司),Sephadex LH-20填料(GE公司),Toyopearl HW-40填料(Toyo Soda MFG公司),色谱纯甲醇(山东禹王有限公司),色谱纯乙腈(迪马公司),分析纯化学试剂(天津富宇精细化工有限公司)。
药材由石家庄以岭药业提供,并由河北以岭医药研究院中药标本室田青存药师鉴定为酸枣Ziziphus jujuba Mill. var. spinosa (Bunge) Hu ex H. F. Chou的成熟干燥种子,标本(2011ZX09201-201-28)保存于暨南大学药学院中药及天然药物研究所。
2 提取与分离酸枣仁14.5 kg 粗碎后,以80 L 70%乙醇提取4次,每次2 h。提取液浓缩后得到浸膏1.78 kg,以10倍量水混悬、离心、滤过后进行D-101大孔树脂柱色谱分离,分别用3倍体积的水及30%、50%、70%、95%乙醇进行洗脱,得到的馏份减压浓缩后,得到5个馏份Fr. A~E。取酸枣仁70%乙醇洗脱部位(SZR-D)69 g通过硅胶柱色谱氯仿-甲醇洗脱得到12个馏份Fr. D1~D12,再取氯仿-甲醇(85∶15)的D9馏份进行ODS柱色谱分离,50%甲醇水洗脱再经HW-40柱色谱及制备型HPLC分离得到化合物5(25.4 mg)。70%甲醇水洗脱得到D9馏份再过HW-40柱色谱,得到的60%甲醇水洗脱下的馏份经HPLC制备得到化合物1(16.5 mg)。取氯仿-甲醇(8∶2)的D10馏份进行ODS柱色谱、HW-40柱色谱和制备型HPLC得到化合物4(4.5 mg)。取氯仿-甲醇(7∶3)的D11馏份进行ODS柱色谱和制备型HPLC得到化合物3(7.7 mg)。取酸枣仁50%乙醇洗脱部位(SZR-C)106.4 g 通过硅胶柱色谱氯仿-甲醇(8∶2)洗脱馏份经过ODS柱色谱和制备型HPLC分别得到化合物2(21.2 mg)。
化合物1的水解及衍生化反应:取化合物1(1.5 mg),加入2 mol/L HCl(2 mL),置于90 ℃水浴中反应2 h。将混合物蒸干,加入1 mL水混悬,再加入等体积的水饱和的氯仿萃取。移除氯仿层,水层蒸干。样品继续进行衍生化实验:加入左旋半胱氨酸甲酯盐酸盐(2.5 mg)于吡啶(1 mL)中溶解,置于60 ℃水浴下反应1 h。再加入邻甲苯基异硫代异氰酸酯(5 μL),继续在60 ℃下反应1 h。反应完成后,直接作为供试品,以进行HPLC分析。单糖对照品(D-葡萄糖、L-葡萄糖各2.0 mg)采用相同的方法进行衍生化反应,反应后直接作为HPLC供试品[12]。
HPLC分析条件:色谱柱:Ultimate TM,XB-C18(250 mm×4.6 mm,5 μm,Welch Materials Inc.)。流动相为25%乙腈-水(含0.1%乙酸);体积流量0.8 mL/min;柱温35 ℃;检测波长250 nm。HPLC分析结果显示化合物1中含有D-葡萄糖(tR=23.02 min)。
3 结构鉴定
化合物1:黄色固体。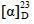 +7.0° (c 0.1,MeOH)。
+7.0° (c 0.1,MeOH)。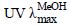 (nm): 213 (4.79),327 (4.79),为黄酮类化合物的紫外特征吸收。ESI-MS给出准分子离子峰m/z: 961 [M+H]+,959 [M-H]-,提示相对分子质量为960。HR-ESI-TOF-MS正离子模式显示m/z: 961.277 3 [M+H]+(C48H49NO21,计算值961.276 6),确定分子式为C48H48O21,不饱和度为25。通过与异斯皮诺素(化合物4)的13C-NMR的数据进行比对(表 1),发现有28个碳信号符合异斯皮诺素的谱学特征。对化合物1进行了酸水解及衍生化,确定化合物1含有D-葡萄糖。而根据糖端基质子的耦合常数δH4.90 (d,J = 10.2 Hz),δH 4.07 (d,J = 7.8 Hz) 确定葡萄糖为β构型。通过1H-1H COSY、HSQC及HMBC谱,对糖基片段的碳氢信号进行了归属。在HMBC谱中(图 1),糖的端基氢信号H-1″与C-7/C-8/C-9存在远程相关,可确定糖基以碳碳单键连在黄酮苷元的C-8位上。H-1′′′和C-2″存在远程相关,提示第2个糖片段连接于上述糖的2″位上。同时,2个糖基的6位H-6″ (δH 4.53,4.16),H-6′′′ (δH 3.89,3.69) 分别和羰基碳信号C-9′′′′′ (δC 166.8) C-9′′′′ (δC 166.5) 相关,提示2个糖基的6位均发生酯化。
(nm): 213 (4.79),327 (4.79),为黄酮类化合物的紫外特征吸收。ESI-MS给出准分子离子峰m/z: 961 [M+H]+,959 [M-H]-,提示相对分子质量为960。HR-ESI-TOF-MS正离子模式显示m/z: 961.277 3 [M+H]+(C48H49NO21,计算值961.276 6),确定分子式为C48H48O21,不饱和度为25。通过与异斯皮诺素(化合物4)的13C-NMR的数据进行比对(表 1),发现有28个碳信号符合异斯皮诺素的谱学特征。对化合物1进行了酸水解及衍生化,确定化合物1含有D-葡萄糖。而根据糖端基质子的耦合常数δH4.90 (d,J = 10.2 Hz),δH 4.07 (d,J = 7.8 Hz) 确定葡萄糖为β构型。通过1H-1H COSY、HSQC及HMBC谱,对糖基片段的碳氢信号进行了归属。在HMBC谱中(图 1),糖的端基氢信号H-1″与C-7/C-8/C-9存在远程相关,可确定糖基以碳碳单键连在黄酮苷元的C-8位上。H-1′′′和C-2″存在远程相关,提示第2个糖片段连接于上述糖的2″位上。同时,2个糖基的6位H-6″ (δH 4.53,4.16),H-6′′′ (δH 3.89,3.69) 分别和羰基碳信号C-9′′′′′ (δC 166.8) C-9′′′′ (δC 166.5) 相关,提示2个糖基的6位均发生酯化。
|
|
表 1 化合物1的1H-NMR和13C-NMR (600/150 MHz,DMSO-d6) 数据 Table 1 1H-NMR and 13C-NMR (600/150 MHz,DMSO-d6) data of compound 1 |
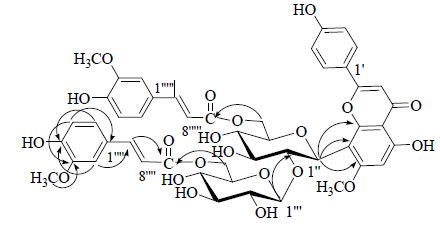 | 图 1 化合物1主要HMBC相关Fig. 1 Key HMBC correlations of compound 1 |
1H-NMR谱中,除异斯皮诺素片段之外,芳香氢信号δH7.31 (1H,d,J = 1.8 Hz),7.09 (1H,dd,J = 8.4,1.8 Hz),6.82 (1H,d,J = 8.4 Hz);7.13 (1H,d,J = 1.8 Hz),6.93 (1H,dd,J = 8.4,1.8 Hz),6.73 (1H,d,J = 8.4 Hz) 相互耦合,提示可能存在2个1,3,4-三取代的苯环;氢信号δH7.48 (1H,d,J = 15.6 Hz),6.28 (1H,d,J = 15.6 Hz);7.47 (1H,d,J = 15.6 Hz),6.31 (1H,d,J = 15.6 Hz) 相互耦合,提示可能存在2对反式双键;高场区还显示氢信号δH3.85 (3H,s),3.72 (3H,s) 提示含有2个甲氧基。13C-NMR谱中,共显示48个碳信号。除异斯皮诺素部分外,还有8个季碳,10个叔碳,2个甲氧基碳共20个信号。通过HSQC将碳氢直接相关归属。在HMBC谱中,H-2′′′′与季碳信号δC 149.4,双键碳信号δC 144.8有相关,可以确定两个碳分别为C-4′′′′和C-7′′′′;H-5′′′′与2个季碳信号δC 147.9和125.6相关,确定两个碳分别为C-3′′′′和C-1′′′′。甲氧基氢信号δC 3.85和C-3′′′′相关,确定甲氧基连接在C-3′′′′上。H-7′′′′与羰基碳信号δC166.5相关,确定双键与C-9′′′′直接相连,由此得到阿魏酰基基团结构。同理可得到另外1个阿魏酰基基团。二者分别连在异斯皮诺素片段的外侧和内侧糖基的6位上。综合上述分析,经文献检索,确定化合物1为1个新的黄酮碳苷化合物,命名为6″,6′′′-二阿魏酰异斯皮诺素。
化合物2:黄色固体。ESI-MS m/z: 749 [M+Na]+,725 [M-H]-。1H-NMR (600 MHz,DMSO-d6) δ: 6.38 (1H,d,J = 1.8 Hz,H-6),6.17 (1H,d,J = 1.8 Hz,H-8),8.02 (1H,d,J = 9.0 Hz,H-2′ 6′),6.88 (1H,d,J = 9.0 Hz,H-3′ 5′),5.57 (1H,d,J = 7.2 Hz,H-1″),5.57 (1H,d,J = 7.2 Hz,H-1″),4.58 (1H,d,J = 7.2 Hz,H-1′′′),4.34 (1H,brs,H-1′′′′);13C-NMR (150 MHz,DMSO-d6)δ: 155.8 (C-2),132.8 (C-3),177.3 (C-4),161.2 (C-5),98.8 (C-6),164.5 (C-7),93.7 (C-8),156.4 (C-9),103.7 (C-10),120.9 (C-1′),130.9 (C-2′ 6′),115.2 (C-3′ 5′),159.9 (C-4′),98.2 (C-1″),81.6 (C-2″),76.7 (C-3″),69.5 (C-4″),75.7 (C-5″),66.1 (C-6″),104.4 (C-1′′′),73.7 (C-2′′′),76.0 (C-3′′′),69.4 (C-4′′′),65.6 (C-5′′′),100.4 (C-1′′′′),70.3 (C-2′′′′),70.6 (C-3′′′′),71.8 (C-4′′′′),68.2 (C-5′′′′),17.6 (C-6′′′′)。以上数据与文献报道一致[13],故鉴定化合物2为山柰酚-3-O-β-D-吡喃木糖基-(1→2)-[α-L-吡喃鼠李糖基-(1→6)]-β-D-吡喃葡萄糖苷。
化合物3:黄色固体。ESI-MS m/z: 631 [M+Na]+,607 [M-H]-。1H-NMR (300 MHz,DMSO-d6) δ: 6.84/6.81 (1H,s,H-3),6.81/6.77 (1H,s,H-8),7.95 (2H,d,J = 8.7 Hz,H-2′,6′),6.89 (2H,d,J = 8.7 Hz,H-3′,5′),3.90 (3H,s,-OCH3),4.68/4.67 (1H,d,J = 9.9 Hz,H-1′′),4.16 (1H,t,J = 7.5 Hz,H-1′′′);13C-NMR (100 MHz,DMSO-d6) δ: 163.7 (C-2),102.7/102.6 (C-3),182.2/181.9 (C-4),159.7 (C-5),108.6/108.5 (C-6),165.0 (C-7),90.7/90.2 (C-8),157.0/156.9 (C-9),104.4/104.1 (C-10),120.2/120.1 (C-1′),128.5 (C-2′,6′),116.2 (C-3′,5′),160.5 (C-4′ ),56.5/56.1 (-OCH3),71.0/70.7 (C-1′′),81.2/80.7 (C-2′′),78.7/78.3 (C-3′′),70.4 (C-4′′),81.9/81.6 (C-5′′),61.5 (C-6′′),105.4/ 105.2 (C-1′′′),74.7/74.5 (C-2′′′),76.6/76.4 (C-3′′′),69.5/69.2 (C-4′′′),76.4/76.3 (C-5′′′),60.6/60.1 (C-6′′′)。以上数据与文献报道一致[3],故鉴定化合物3为斯皮诺素。
化合物4:淡黄色粉末。ESI-MS m/z: 631 [M+Na]+,643 [M+Cl]-。1H-NMR (400 MHz,DMSO-d6) δ: 6.47 (1H,s,H-3),6.78 (1H,s,H-6),8.02 (2H,d,J = 8.7 Hz,H-2′,6′),6.89 (2H,d,J = 8.7 Hz,H-3′,5′),3.87 (3H,s,-OCH3),4.83 (1H,d,J = 9.9 Hz,H-1″),4.05 (1H,t,J = 9.3 Hz,H-1′′′);13C-NMR (100 MHz,DMSO-d6) δ: 163.4 (C-2),102.5 (C-3),182.2 (C-4),161.5 (C-5),95.1 (C-6),164.2 (C-7),104.7 (C-8),155.3 (C-9),104.2 (C-10),121.3 (C-1′),129.0 (C-2′,6′),115.9 (C-3′,5′),161.6 (C-4′),56.6 (-OCH3),71.4 (C-1″),80.9 (C-2″),78.2 (C-3″),70.1 (C-4″),81.8 (C-5″),60.9 (C-6″),104.9 (C-1′′′),74.5 (C-2′′′),76.1 (C-3′′′),69.4 (C-4′′′),76.1 (C-5′′′),60.2 (C-6′′′)。以上数据与文献报道一致[3],故鉴定化合物4为异斯皮诺素。
化合物5:黄色粉末。ESI-MS m/z: 807 [M+Na]+,783 [M-H]-。1H-NMR (400 MHz,DMSO-d6) δ: 6.71/6.69 (1H,s,H-3),6.67/6.53 (1H,s,H-8),7.81 (2H,d,J = 8.8 Hz,H-2′,6′),6.90/6.85 (2H,d,J = 8.8 Hz,H-3′,5′),4.70/4.68 (1H,d,J = 10.0 Hz,H-1′′),4.25 (1H,t,J = 9.2 Hz,H-1′′′),7.18/7.05 (1H,d,J = 1.2 Hz,H-2′′′′),6.78/6.74 (1H,d,J = 8.0 Hz,H-5′′′′),6.93/6.79 (1H,dd,J = 8.0,1.2 Hz,H-6′′′′),7.22/7.09 (1H,d,J = 15.6 Hz,H-7′′′′),6.24/6.16 (1H,d,J = 15.6 Hz,H-8′′′′);13C-NMR (100 MHz,DMSO-d6) δ: 163.9/ 163.6 (C-2),103.1/102.9 (C-3),182.3/181.8 (C-4),160.8/159.5 (C-5),108.7 (C-6),165.2/164.2 (C-7),90.6/89.9 (C-8),157.0/156.9 (C-9),104.5/104.0 (C-10),121.2/121.1 (C-1′),128.6/128.5 (C-2′,6′),115.9/115.8 (C-3′,5′),161.3 (C-4′ ),56.4/56.1 (-OCH3),71.0/70.7 (C-1′′),81.7/80.2 (C-2′′),78.8/78.6 (C-3′′),70.4/70.3 (C-4′′),82.0 (C-5′′),61.5 (C-6′′),105.7/105.2 (C-1′′′),74.5/74.4 (C-2′′′),76.3 (C-3′′′),68.9/68.7 (C-4′′′),73.4/73.2 (C-5′′′),62.4/62.1 (C-6′′′),125.5/125.3 (C-1′′′′),110.9/110.8 (C-2′′′′),147.9 (C-3′′′′),149.3 (C-4′′′′),115.4 (C-5′′′′),123.2/123.0 (C-6′′′′),144.8/ 144.7 (C-7′′′′),114.1/113.6 (C-8′′′′),166.3/166.2 (C-9′′′′),55.7/55.6 (-OCH3)。以上数据与文献报道一致[3],故鉴定化合物5为6′′′-阿魏酰斯皮诺素。
| [1] | 中国药典 [S]. 一部. 2010. |
| [2] | Otsuka H, Akiyama T, Kawai K I. The structure of jujubosides A and B, the saponins isolated from the seeds of Zizyphus jujuba [J]. Phytochemistry, 1978, 17(8): 1349-1352. |
| [3] | Cheng G, Bai Y J, Zhao Y Y. Flavonoids from Ziziphus jujuba Mill. var. spinosa [J]. Tetrahedron, 2000, 56(45): 8915-8920. |
| [4] | Zhang L, Xu Z L, Wu C F. Two new flavonoid glycosides from Ziziphi Spinosae Semen [J]. J Asian Nat Prod Res, 2012, 14(2): 121-128. |
| [5] | Xie Y Y, Xu Z L, Wang H. A novel spinosin derivative from Ziziphi Spinosae Semen [J]. J Asian Nat Prod Res, 2011, 13(12): 1151-1157. |
| [6] | Wang Y, Dai Y, Yao X S. New triterpene glycosides from Ziziphi Spinosae Semen [J]. Fitoterapia, 2013, 90: 185-191. |
| [7] | Yoshikawa M, Murakami T, Ikebata A. Bioactive saponins and glycosides. X. on the constituents of Ziziphi Spinosae Semen, the seeds of Zizyphus jujuba Mill. var. spinosa Hu (1): structures and histamine release- inhibitory effect of jujubosides A1 and C and acetyljujuboside B [J]. Chem Pharm Bull, 1997, 45(7): 1186-1192. |
| [8] | Matsuda H, Murakami T, Ikebata A. Bioactive saponins and glycosides XIV. Structure elucidation and immunological adjuvant activity of novel protojujubogenin type triterpene bisdesmosides, protojujubosides A, B, and B1, from the seeds of Zizyphus jujuba var. spinosa [J]. Chem Pharm Bull, 1999, 47(12): 1744-1748. |
| [9] | Park M H, Suh D Y, Han B H. Absolute configuration of a cyclopeptide alkaloid, sanjoinine-G1, from Zizyphus vulgaris var. spinosus [J]. Phytochemistry, 1996, 43(3): 701-704. |
| [10] | Han B H, Park M H, Han Y N. Aporphine and tetrahydrobenzylisoquinoline alkaloids from the seeds of Zizyphus vulgaris var. spinosus [J]. Arch Pharm Res, 1989, 12(4): 263-268. |
| [11] | Jiang J G, Huang X J, Chen J. Comparison of the sedative and hypnotic effects of flavonoids, saponins, and polysaccharides extracted from Semen Ziziphus jujube [J]. Nat Prod Res, 2007, 21(4): 310-320. |
| [12] | Tanaka T, Nakashima T, Ueda T. Facile discrimination of aldose enantiomers by reversed-phase HPLC [J]. Chem Pharm Bull, 2007, 55(6): 899-901. |
| [13] | Gao D F, Xu M, Zhao P. Kaempferol acetylated glycosides from the seed cake of Camellia oleifera [J]. Food Chem, 2011, 124(2): 432-436. |
 2014, Vol. 45
2014, Vol. 45


