兔儿风属Ainsliaea DC. 隶属于菊科(Compositae)帚菊木族(Mutisieae Cass.),全世界约有70种,分布于亚洲东南部。我国有44种4个变种,除1种产于东北外,其余均产于长江流域及其以南各省[1]。到目前为止,从本属植物分到的化合物主要有倍半萜内酯及其苷、三萜、甾体和黄酮等[2]。宽叶兔儿风Ainsliaea latifolia (D. Don) Sch. -Bip. 为菊科兔儿风属植物,分布于我国西藏、云南、四川、贵州、广西及海南等地,民间主要用于治疗风寒咳嗽、肠炎、痢疾、跌打损伤等[3]。目前尚未有关于该植物化学成分及生物活性方面的研究报道。为了从中寻找更多结构新颖的生物活性成分,本实验对宽叶兔儿风的化学成分进行了研究,从其全草80%乙醇提取物中得到了13个化合物,分别鉴定为木栓酮(friedelin,1)、蒲公英萜醇乙酸酯(taraxeryl acetate,2)、3β-hydroxy-11α,12α-epoxy-friedoolean- 14-ene(3)、careborin(4)、cis-careborin(5)、3α-E-feruloyltaraxerol(6)、3α-Z-feruloyltaraxerol(7)、3-oxo-11α-methoxyolean-12-ene(8)、diaspanolide A(9)、diaspanolide B(10)、ainsliaolide A(11)、豆甾醇(stigmasterol,12)、β-谷甾醇(β-sitosterol,13)。化合物1~13均为首次从该植物中分离得到,其中包括8个三萜类(1~8),3个倍半萜类(9~11),2个甾体类化合物(12~13)。部分结构见图 1。
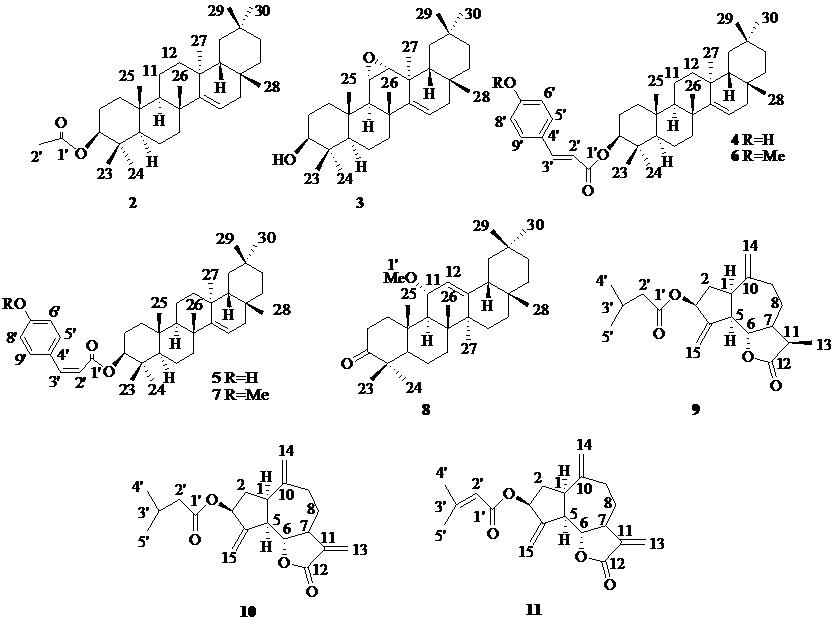 | 图 1 化合物2~11的结构 Fig. 1 Structures of compounds 2-11 |
Bruker DRX—500型核磁共振谱仪(德国Bruker公司);Agilent LC/MSD Trap XCT质谱仪(美国Agilent公司);SK5200H型超声发生器(上海科导超声仪器有限公司);Sephadex LH-20(Pharmacia公司);反相硅胶C18(Merck公司);薄层色谱硅胶和HSGF254硅胶预制板(烟台江友硅胶开发有限公司);提取用乙醇为工业级,质谱用试剂为色谱级,其余试剂均为分析纯。
宽叶兔儿风全草于2013年9月采自贵州贵阳,经贵阳医学院生药教研室龙庆德教授鉴定为宽叶兔儿风Ainsliaea latifolia (D. Don) Sch. -Bip.,植物标本(20130905)现保存在第二军医大学药学院天然药化教研室。
2 提取与分离宽叶兔儿风全草(15 kg)粉碎后用80%乙醇浸泡4次,每次48 h,提取液回流蒸干后得80%乙醇提取物2 kg。依次用石油醚、氯仿、醋酸乙酯、甲醇进行固相萃取,取氯仿部位(105 g)进行硅胶柱色谱,石油醚-醋酸乙酯(100∶1→50∶1→30∶1→10∶1→5∶1→0∶1)梯度洗脱,得到7个流分(Fr. 1~7)。其中Fr. 2(21 g)经硅胶柱分离,以石油醚- 丙酮(50∶1→0∶1)梯度洗脱,再经Sephadex LH-20 柱色谱与制备薄层分离纯化得到化合物1(1.6 g)、2(24.5 mg)、12(75.6 mg)、13(43.8 mg)。Fr. 3(17 g)用硅胶柱分离,以石油醚-醋酸乙酯(50∶1→0∶1)进行梯度洗脱,再经Sephadex LH-20柱色谱与制备薄层分离纯化得到化合物9(43.6 mg)、10(350 mg)、11(11.6 mg)。Fr. 5(13 g)用开放硅胶柱分离,以石油醚-醋酸乙酯(50∶1→0∶1)进行梯度洗脱,再经Sephadex LH-20柱色谱、制备薄层分离纯化得到化合物3(2.3 mg)、8(2.4 mg)。Fr. 6(26 g)用ODS柱色谱分离,以甲醇-水进行梯度洗脱,再经Sephadex LH-20柱色谱、制备薄层、高压液相制备分离纯化得到化合物4(6.6 mg)、5(6.0 mg)、6(15 mg)、7(44 mg)。
3 结构鉴定化合物1:白色固体,ESI-MS m/z: 449 [M+Na]+,425 [M-H]-,分子式为C30H50O。1H-NMR (500 MHz,CDCl3) δ: 2.39 (1H,m,H-2),2.26 (2H,m,H-2,4),1.95 (1H,m,H-1),1.75 (1H,m,H-6),1.66 (1H,m,H-1),1.17 (3H,s,H-28),1.04 (3H,s,H-27),1.00 (3H,s,H-26),0.99 (3H,s,H-27),0.94 (3H,s,H-29),0.87 (3H,d,J = 6.6 Hz,H-23),0.86 (3H,s,H-25),0.71 (3H,s,H-24);13C-NMR (125 MHz,CDCl3) δ: 22.2 (C-1),41.5 (C-2),213.1 (C-3),58.2 (C-4),42.1 (C-5),41.3 (C-6),18.2 (C-7),53.1 (C-8),37.4 (C-9),59.4 (C-10),35.6 (C-11),30.5 (C-12),39.7 (C-13),38.3 (C-14),32.4 (C-15),36.0 (C-16),30.0 (C-17),42.8 (C-18),35.3 (C-19),28.1 (C-20),32.7 (C-21),39.2 (C-22),6.8 (C-23),14.6 (C-24),17.9 (C-25),20.2 (C-26),18.6 (C-27),32.1 (C-28),35.0 (C-29),31.8 (C-30)。以上数据与文献报道一致[4],故鉴定化合物1为木栓酮。
化合物2:白色固体,ESI-MS m/z: 491 [M+Na]+,467 [M-H]-,分子式为C32H52O2。1H-NMR (500 MHz,CDCl3) δ: 5.52 (1H,dd,J = 8.2,3.2 Hz,H-15),4.45 (1H,dd,J = 10.6,5.6 Hz,H-3),2.04 (3H,s,H-2′),1.09 (3H,s,H-26),0.95 (6H,s,H-24,29),0.91 (6H,s,H-30,27),0.87 (3H,s,H-23),0.86 (3H,s,H-25),0.82 (3H,s,H-28);13C-NMR (125 MHz,CDCl3) δ: 37.9 (C-1),25.9 (C-2),81.0 (C-3),37.7 (C-4),55.6 (C-5),18.7 (C-6),33.1 (C-7),39.0 (C-8),49.2 (C-9),37.5 (C-10),17.5 (C-11),35.8 (C-12),36.6 (C-13),158.0 (C-14),116.9 (C-15),33.3 (C-16),35.1 (C-17),48.7 (C-18),41.2 (C-19),29.7 (C-20),33.7 (C-21),37.4 (C-22),28.8 (C-23),16.6 (C-24),15.5 (C-25),28.0 (C-26),29.8 (C-27),29.9 (C-28),23.4 (C-29),21.3 (C-30),171.0 (C-1′),21.3 (C-2′)。以上数据与文献报道一致[5],故鉴定化合物2为蒲公英萜醇乙酸酯。
化合物3:白色固体;ESI-MS m/z: 463 [M+Na]+,439 [M-H]-;分子式为C30H48O2。1H-NMR (500 MHz,CDCl3) δ: 5.55 (1H,dd,J = 8.1,3.0 Hz,H-15),4.12 (1H,d,J = 7.1 Hz,H-3),3.25 (1H,m,H-11),3.12 (1H,t,J = 5.1 Hz,H-12),2.81 (1H,d,J = 4.6 Hz,H-9),1.08 (6H,s,H-25,26),1.01 (3H,s,H-24),0.99 (3H,s,H-29),0.97 (3H,s,H-27),0.86 (3H,s,H-23),0.82 (3H,s,H-28,30);13C-NMR (125 MHz,CDCl3) δ: 38.2 (C-1),26.8 (C-2),79.0 (C-3),38.6 (C-4),54.6 (C-5),18.9 (C-6),33.1 (C-7),38.9 (C-8),52.0 (C-9),37.5 (C-10),53.6 (C-11),58.3 (C-12),36.6 (C-13),157.1 (C-14),118.9 (C-15),35.2 (C-16),35.4 (C-17),48.1 (C-18),40.3 (C-19),28.7 (C-20),36.5 (C-21),38.2 (C-22),27.9 (C-23),16.9 (C-24),15.4 (C-25),27.0 (C-26),30.2 (C-27),29.9 (C-28),33.6 (C-29),19.5 (C-30)。以上数据与文献报道一致[6],故鉴定化合物3为3β-hydroxy-11α,12α- epoxy-friedoolean-14-ene。
化合物4:白色固体,ESI-MS m/z: 595 [M+Na]+,571 [M-H]-,分子式为C39H56O3。1H-NMR (500 MHz,CDCl3) δ: 7.60 (1H,d,J = 15.9 Hz,H-3′),7.43 (2H,d,J = 8.5 Hz,H-6′,8′),6.84 (2H,d,J = 8.5 Hz,H-5′,9′),6.30 (1H,d,J = 15.9 Hz,H-2′),5.54 (1H,dd,J = 8.1,3.1 Hz,H-15),4.60 (1H,dd,J = 10.9,5.4 Hz,H-3),1.10 (3H,s,H-26),0.98 (3H,s,H-25),0.95 (6H,s,H-24,29),0.91 (6H,s,H-30,27),0.90 (3H,s,H-23),0.82 (3H,s,H-28);13C-NMR (125 MHz,CDCl3) δ: 37.5 (C-1),23.6 (C-2),81.0 (C-3),39.0 (C-4),55.6 (C-5),18.7 (C-6),33.1 (C-7),37.7 (C-8),49.2 (C-9),37.4 (C-10),17.5 (C-11),36.6 (C-12),37.9 (C-13),158.0 (C-14),116.9 (C-15),33.7 (C-16),35.8 (C-17),48.7 (C-18),41.2 (C-19),28.8 (C-20),35.1 (C-21),37.9 (C-22),28.0 (C-23),16.8 (C-24),15.5 (C-25),25.9 (C-26),29.9 (C-27),29.8 (C-28),33.3 (C-29),21.3 (C-30),167.4 (C-1′),116.2 (C-2′),144.0 (C-3′),127.3 (C-4′),129.9 (C-5′,9′),115.8 (C-6′,8′),157.6 (C-7′)。以上数据与文献报道一致[7],故鉴定化合物4为careborin。
化合物5:白色固体,ESI-MS m/z: 595 [M+Na]+,571 [M-H]-,分子式为C39H56O3。1H-NMR (500 MHz,CDCl3) δ: 7.61 (2H,d,J = 7.4 Hz,H-6′,8′),6.84 (1H,d,J = 12.7 Hz,H-3′),6.77 (2H,d,J = 8.6 Hz,H-5′,9′),5.84 (1H,dd,J = 8.1,12.6 Hz,H-2′),5.54 (1H,dd,J = 8.1,3.2 Hz,H-15),4.52 (1H,dd,J = 11.0,4.9 Hz,H-3),1.09 (3H,s,H-26),0.96 (6H,s,H-24,29),0.91 (6H,s,H-30,27),0.87 (3H,s,H-25),0.85 (3H,s,H-23),0.82 (3H,s,H-28);13C-NMR (125 MHz,CDCl3) δ: 37.5 (C-1),23.4 (C-2),81.2 (C-3),39.0 (C-4),55.6 (C-5),18.7 (C-6),33.1 (C-7),37.7 (C-8),49.1 (C-9),37.4 (C-10),17.5 (C-11),36.6 (C-12),37.9 (C-13),158.0 (C-14),116.9 (C-15),33.7 (C-16),35.8 (C-17),48.8 (C-18),41.2 (C-19),28.8 (C-20),35.1 (C-21),37.9 (C-22),28.0 (C-23),16.6 (C-24),15.5 (C-25),25.9 (C-26),29.9 (C-27),29.8 (C-28),33.3 (C-29),21.3 (C-30),166.7 (C-1′),116.9 (C-2′),143.4 (C-3′),127.4 (C-4′),132.2 (C-5′,9′),115.1 (C-6′,8′),156.8 (C-7′)。以上数据与文献报道一致[8],故鉴定化合物5为cis-careborin。
化合物6:白色固体,ESI-MS m/z: 625 [M+Na]+,601 [M-H]-,分子式为C40H58O4。1H-NMR (500 MHz,CDCl3) δ: 7.59 (1H,d,J = 15.9 Hz,H-3′),7.08 (1H,dd,J = 8.0,1.5 Hz,H-9′),7.06 (1H,d,J = 1.5 Hz,H-5′),6.91 (1H,d,J = 8.1 Hz,H-8′),6.29 (1H,d,J = 15.9 Hz,H-2′),5.57 (1H,dd,J = 8.1,3.2 Hz,H-15),4.52 (1H,dd,J = 11.0,4.9 Hz,H-3),3.92 (3H,s,H-10′),1.10 (3H,s,H-26),0.98 (3H,s,H-25),0.95 (6H,s,H-24,29),0.91 (6H,s,H-30,27),0.90 (3H,s,H-23),0.82 (3H,s,H-28);13C-NMR (125 MHz,CDCl3) δ: 37.5 (C-1),23.5 (C-2),80.7 (C-3),39.0 (C-4),55.7 (C-5),18.7 (C-6),33.1 (C-7),37.7 (C-8),49.1 (C-9),37.4 (C-10),17.5 (C-11),36.6 (C-12),37.9 (C-13),158.0 (C-14),116.9 (C-15),33.7 (C-16),35.8 (C-17),48.8 (C-18),41.2 (C-19),28.8 (C-20),35.1 (C-21),37.9 (C-22),28.0 (C-23),16.6 (C-24),15.5 (C-25),25.9 (C-26),29.9 (C-27),29.8 (C-28),33.3 (C-29),21.3 (C-30),167.1 (C-1′),116.9 (C-2′),143.3 (C-3′),127.1 (C-4′),109.2 (C-5′),146.7 (C-6′),147.8 (C-7′),114.6 (C-8′),123.0 (C-9′),56.0 (C-10′)。以上数据与文献报道一致[9],故鉴定化合物6为3α-E- feruloyltaraxerol。
化合物7:白色固体,ESI-MS m/z: 625 [M+Na]+,601 [M-H]-,分子式为C40H58O3。1H-NMR (500 MHz,CDCl3) δ: 7.78 (1H,d,J = 1.8 Hz,H-5′),7.10 (1H,dd,J = 8.2,1.7 Hz,H-9′),6.87 (1H,d,J = 8.2 Hz,H-8′),6.77 (1H,d,J = 13 Hz,H-3′),5.82 (1H,d,J = 14.7 Hz,H-2′),5.54 (1H,dd,J = 8.1,3.2 Hz,H-15),4.52 (1H,dd,J = 11.0,4.9 Hz,H-3),3.92 (3H,s,H-10′),1.10 (3H,s,H-26),0.98 (3H,s,H-25),0.95 (6H,s,H-24,29),0.91 (6H,s,H-30,27),0.90 (3H,s,H-23),0.82 (3H,s,H-28);13C-NMR (125 MHz,CDCl3) δ: 37.5 (C-1),23.5 (C-2),80.7 (C-3),39.0 (C-4),55.7 (C-5),18.7 (C-6),33.1 (C-7),37.7 (C-8),49.1 (C-9),37.4 (C-10),17.5 (C-11),36.6 (C-12),37.9 (C-13),158.0 (C-14),116.9 (C-15),33.7 (C-16),35.8 (C-17),48.8 (C-18),41.2 (C-19),28.8 (C-20),35.1 (C-21),37.9 (C-22),28.0 (C-23),16.6 (C-24),15.5 (C-25),25.9 (C-26),29.9 (C-27),29.8 (C-28),33.3 (C-29),21.3 (C-30),166.5 (C-1′),116.9 (C-2′),143.3 (C-3′),127.3 (C-4′),112.8 (C-5′),145.9 (C-6′),146.9 (C-7′),113.8 (C-8′),125.5 (C-9′),56.0 (C-10′)。以上数据与文献报道一致[9],故鉴定化合物7为3α-Z- feruloyltaraxerol。
化合物8:无色油状物,ESI-MS m/z: 477 [M+Na]+,453 [M-H]-,分子式为C31H50O2。1H-NMR (500 MHz,CDCl3) δ: 5.36 (1H,d,J = 3.1 Hz,H-12),3.93 (1H,dd,J = 9.4,3.1 Hz,H-11),3.24 (3H,s,H-1′),1.22 (3H,s,H-27),1.15 (3H,s,H-25),1.11 (3H,s,H-23),1.07 (3H,s,H-24),1.06 (3H,s,H-26),0.90 (3H,s,H-30),0.89 (3H,s,H-29),0.84 (3H,s,H-28);13C-NMR (125 MHz,CDCl3) δ: 40.3 (C-1),34.4 (C-2),218.1 (C-3),47.7 (C-4),55.5 (C-5),19.7 (C-6),32.9 (C-7),42.9 (C-8),50.3 (C-9),37.7 (C-10),76.3 (C-11),121.6 (C-12),149.3 (C-13),42.0 (C-14),26.2 (C-15),26.8 (C-16),32.4 (C-17),47.2 (C-18),46.4 (C-19),31.1 (C-20),34.7 (C-21),36.9 (C-22),26.7 (C-23),21.5 (C-24),16.4 (C-25),18.1 (C-26),25.1 (C-27),28.5 (C-28),33.2 (C-29),23.6 (C-30),53.7 (C-1′)。以上数据与文献报道一致[10],故鉴定化合物8为3-oxo-11α-methoxyolean-12-ene。
化合物9:黄色油状物,ESI-MS m/z: 355 [M+Na]+,331 [M-H]-,分子式为C20H28O4。1H-NMR (500 MHz,CDCl3) δ: 5.54 (1H,m,H-3),5.39 (1H,t,J = 2.0 Hz,H-15),5.25 (1H,t,J = 2.0 Hz,H-15),4.89 (2H,d,J = 9.0 Hz,H-14),4.08 (1H,t,J = 9.8 Hz,H-6),2.89 (1H,m,H-1),2.83 (1H,m,H-5),2.67 (1H,m,H-7),2.46 (3H,m,H-2,9),1.15 (3H,d,J = 7.8 Hz,H-13),0.96 (6H,d,J = 6.6 Hz,H-4′,5′);13C-NMR (125 MHz,CDCl3) δ: 43.7 (C-1),36.2 (C-2),74.4 (C-3),148.9 (C-4),50.1 (C-5),83.7 (C-6),45.7 (C-7),28.7 (C-8),36.2 (C-9),148.4 (C-10),39.2 (C-11),179.6 (C-12),11.4 (C-13),113.4 (C-14),113.2 (C-15),172.8 (C-1′),43.6 (C-2′),25.7 (C-3′),22.4 (C-4′),22.4 (C-5′)。以上数据与文献报道一致[11],故鉴定化合物9为diaspanolide A。
化合物10:黄色油状物,ESI-MS m/z: 353 [M+Na]+,329 [M-H]-,分子式为C20H26O4。1H-NMR (500 MHz,CDCl3) δ: 6.21 (1H,d,J = 3.5 Hz,H-13),5.56 (1H,m,H-3),5.49 (1H,d,J = 3.1 Hz,H-13),5.45 (1H,t,J = 2.0 Hz,H-15),5.27 (1H,t,J = 2.1 Hz,H-15),4.97 (2H,d,J = 6.4 Hz,H-14),4.06 (1H,dd,J = 16.9,7.6 Hz,H-6),2.94 (1H,m,H-1),2.85 (2H,m,H-5,7),2.46 (2H,m,H-9),1.78 (1H,m,H-2),0.96 (6H,d,J = 6.6 Hz,H-4′,5′);13C-NMR (125 MHz,CDCl3) δ: 44.6 (C-1),34.6 (C-2),74.3 (C-3),147.8 (C-4),50.2 (C-5),83.9 (C-6),45.2 (C-7),30.6 (C-8),36.6 (C-9),148.2 (C-10),139.5 (C-11),170.0 (C-12),120.3 (C-13),114.3 (C-14),113.4 (C-15),172.8 (C-1′),43.6 (C-2′),25.8 (C-3′),22.4 (C-4′),22.4 (C-5′)。以上数据与文献报道一致[12],故鉴定化合物10为diaspanolide B。
化合物11:黄色油状物,ESI-MS m/z: 351 [M+Na]+,327 [M-H]-,分子式为C20H24O4。1H-NMR (500 MHz,CDCl3) δ: 6.22 (1H,d,J = 3.5 Hz,H-13),5.72 (1H,m,H-2′),5.59 (1H,m,H-3),5.49 (1H,d,J = 3.1 Hz,H-13),5.47 (1H,t,J = 2.0 Hz,H-15),5.30 (1H,t,J = 2.0 Hz,H-15),4.97 (2H,d,J = 12.7 Hz,H-14),4.07 (1H,dd,J = 16.9,7.6 Hz,H-6),2.94 (1H,m,H-1),2.85 (2H,m,H-5,7),2.46 (2H,m,H-9),2.19 (3H,d,J = 0.9 Hz,H-5′),1.91 (3H,d,J = 1.0 Hz,H-4′),1.78 (1H,m,H-2),0.96 (6H,d,J = 6.6 Hz,H-4′,5′);13C-NMR (125 MHz,CDCl3) δ: 44.7 (C-1),34.6 (C-2),73.7 (C-3),147.9 (C-4),50.3 (C-5),84.0 (C-6),45.2 (C-7),30.7 (C-8),36.8 (C-9),148.4 (C-10),139.6 (C-11),170.0 (C-12),120.3 (C-13),114.4 (C-14),113.2 (C-15),166.3 (C-1′),115.9 (C-2′),157.4 (C-3′),20.3 (C-4′),27.4 (C-5′)。以上数据与文献报道一致[13],故鉴定化合物11为ainsliaolide A。
化合物12:白色固体,ESI-MS m/z: 435 [M+Na]+,411 [M-H]-,分子式为C29H48O。1H-NMR (500 MHz,CDCl3) δ: 5.36 (1H,d,J = 5.2 Hz,H-6),5.14 (1H,m,H-22),5.01 (1H,m,H-23),3.53 (1H,m,H-3),0.82 (3H,t,J = 7.2 Hz,H-29),0.71 (3H,s,H-18);13C-NMR (125 MHz,CDCl3) δ: 37.2 (C-1),31.7 (C-2),71.8 (C-3),42.3 (C-4),140.7 (C-5),121.7 (C-6),31.9 (C-7),31.9 (C-8),50.2 (C-9),36.5 (C-10),21.1 (C-11),39.7 (C-12),42.2 (C-13),55.9 (C-14),24.4 (C-15),28.9 (C-16),56.9 (C-17),12.0 (C-18),19.4 (C-19),40.5 (C-20),21.2 (C-21),138.3 (C-22),129.3 (C-23),51.2 (C-24),31.9 (C-25),19.0 (C-26),21.1 (C-27),25.4 (C-28),12.2 (C-29)。以上数据与文献报道一致[14],故鉴定化合物12为豆甾醇。
化合物13:白色固体,ESI-MS m/z: 437 [M+Na]+,413 [M-H]-,分子式为C29H50O。1H-NMR (500 MHz,CDCl3) δ: 5.36 (1H,m,H-6),3.53 (1H,m,H-3),1.02 (3H,s,H-19),0.92 (3H,d,J = 6.6 Hz,H-21);13C-NMR (125 MHz,CDCl3) δ: 37.2 (C-1),31.7 (C-2),71.8 (C-3),42.3 (C-4),140.8 (C-5),121.7 (C-6),31.9 (C-7),31.9 (C-8),50.1 (C-9),36.5 (C-10),21.1 (C-11),39.8 (C-12),42.3 (C-13),56.8 (C-14),24.3 (C-15),28.2 (C-16),56.0 (C-17),11.8 (C-18),19.4 (C-19),36.1 (C-20),18.8 (C-21),33.9 (C-22),26.1 (C-23),45.8 (C-24),29.1 (C-25),19.8 (C-26),19.0 (C-27),23.1 (C-28),12.0 (C-29)。以上数据与文献报道一致[15],故鉴定化合物13为β-谷甾醇。
| [1] | 中国科学院中国植物志编辑委员会. 中国植物志 [M]. 北京: 科学出版社, 1996. |
| [2] | 徐希科, 柳润辉, 李慧梁, 等. 兔耳风属植物的化学和药理研究进展 [J]. 药学实践杂志, 2009, 27(4): 245-247. |
| [3] | 江苏新医学院. 中药大辞典 [M]. 上海: 上海人民出版社, 1977. |
| [4] | Toshihiro A, Kazuhiro Y, Toshitake T, et al. Triterpenoid ketones from Lingnania chungii Mcclure: arborinone, friedin and glutinone [J]. Chem Pharm Bull, 1992, 40(3): 789-791. |
| [5] | 汪 毅, 李 铣, 孟大利, 等. 蓝刺头化学成分的研究 [J]. 中草药, 2006, 37(2): 189-190. |
| [6] | Syed A I, Muhammad S A. Constituents of Nepeta crassifolia [J]. Fitoterepia, 1989, 60: 351-352. |
| [7] | Talapatra B, Basak A, Talapatra S K. Terpenoids and related compounds: part XX. careaborin, a new triterpene ester from the leaves of Careya arborea [J]. J Indian Chem Soc, 1981, 58: 814-815. |
| [8] | Kokplo U, Chavasiri W, Chittawong V, et al. Taraxery 1 cis-p-hydroxy cinnamate, a novel taraxery l from Rhizophora apiculata [J]. J Nat Prod, 1990, 53(4): 953-955. |
| [9] | Surat L, Chatchanok K, Chanita P, et al. Pentacyclic triterpenoid esters from the fruits of Bruguiera cylindrical [J]. J Nat Prod, 2004, 67: 886-888. |
| [10] | Maria T R de A, Carla R L, Jose M P, et al. Antiproliferative terpenoids and alkaloids from the roots of Maytenus vitisidaea and Maytenus spinosa [J]. Phytochemistry, 2010, 71(14/15): 1741-1748. |
| [11] | Adegawa S, Miyase T, Ueno A. Sesquiterpene lactones from Diaspananthus uniflorus (Sch. -Bip.) Kitam [J]. Chem Pharm Bull, 1987, 35: 1479-1485. |
| [12] | Rachel M, Isabel R C, Blanca R C, et al. Sesquiterpene lactones and phenylpropanoids from Cosmos pringlei [J]. J Nat Prod, 2002, 65: 1030-1032. |
| [13] | Pu J X, Zhan J F, Yang X D, et al. A new sesquiterpene lactones from Ainsliaea bonatii [J]. Chin Chem Lett, 2004, 15(12): 1451-1456. |
| [14] | 肖炳坤, 黄荣清, 杨建云, 等. 山栀茶化学成分研究 [J]. 中草药, 2011, 42(10): 1948-1951. |
| [15] | 周先礼, 秦长红, 梅 莹, 等. 髯花杜鹃叶的化学成分研究 [J]. 中草药, 2011, 41(2): 206-208. |
 2014, Vol. 45
2014, Vol. 45


