2. State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650204, China
Cynanchum otophyllum Schneid., Qingyangshen in Chinese, is a species of Cynanchum L.(Asclepiadaceae), and a Chinese herbal medicine(CHM)distributed extensively over Southwest China. Pharmacodynamic and clinical experiments have established that the chloroform extract and ethyl acetate extract from the rhizome of C. otophyllum were especially effective against epilepsy and chronic hepatitis(Pei et al, 1981; 1987; Zhou, 1991). Since 1984, Qingyangshen Tablets(containing the total glycosides of C. otophyllum)have been manufactured by Lijiang Pharmaceutical Co., Yunnan Baiyao Group, China. The steroidal constituents from the plants of Cynanchum L. have been reported(Hayashi and Mitsuhashi, 1972). From the rhizome of C. otophyllum, researchers isolated nine constituents including two C21 steroidal glycosides(Mu and Zhou, 1983a; 1983b; Mu et al, 1986). Consequently, Mu et al(1986)developed C. otophyllum into three novel medicines(Patents of China: ZL 98 1 18938.5, ZL 98 1 18173.2, and ZL 96 1 11270.0). The authors carried out further important investigations. However, most compounds in total glycosides were difficult to separate. To study these compounds, the authors used the acidic hydrolysis reaction universal in the research on glycosides to obtain secondary glycosides that are easy to separate. Moreover, some glycosides were easy to separate after other glycosides changed to secondary glycosides. From the ethyl acetate extract(total glycosides) and its acidic hydrolysis part of the rhizome of C. otophyllum, the authors isolated seven new carbohydrates(Zhao et al, 2007; 2008a) and three new C21 steroidal glycosides(Zhao et al, 2005; 2008b). Seven compounds were isolated from the rhizome(Zhao et al, 2009). Furthermore, this article reports three new C21 steroidal glycosides obtained from the acidic hydrolysis part, such as deacetylmetaplexigenin 3-O-β-D-ole and ropyranosyl-(1→4)- α-D-ole and ropyranosyl-(1→4)-α-D-ole and ropyranoside(1), deacetylmetaplexigenin 3-O-α-D-ole and ropyranosyl-(1→4)- β-D-thevetopyranosyl-(1→4)-α-D-ole and ropyranoside(2), and deacetylmetaplexigenin 3-O-β-D-cymaropyranosyl-(1→4)-α- D-ole and ropyranoside(3). Considering their structures, compounds 1 and 2 might be native glycosides, and compound 3 might be a native glycoside or artificial product as a fragment of corresponding native glycoside.
2. Materials and methods 2.1 GeneralMelting points were determined on a WC-1 Micromelting Point Apparatus(uncorrected, Instrument Plant of Sichuan University, China). Optical rotations were measured on a Horiba Sepa-300 Digital Polarimeter(Japan). The IR spectra were measured on a Perkin-Elmer 577 Spectrophotometer(USA). The UV spectra were measured on a Shimadzu Double-beam 210A Spectrometer(Japan). FAB-MS was performed on a VG AutoSpec-3000 Spectrometer(UK). Bruker Am-400 and DRX-500 instruments(USA)were used to record 1H-NMR, 2D NMR(400 MHz), and 13C-NMR. C5D5N was the solvent and the internal st and ard was at room temperature. Column chromatography(CC)was carried out on silica gel. Silica gel(200-300 mesh)for CC and silica gel plate(GF-254)for thin-layer chromatography(TLC)were the products of Qingdao Haiyang Chemical Group Co., China.
2.2 Plant materialsThe rhizome of Cynanchum otophyllum Schneid. was bought from a drug market in Kunming. It was identified by Dr. Yue-mao Shen and a voucher specimen(KUN No. 0776933)was deposited in the Herbarium of Kunming Institute of Botany, Chinese Academy of Sciences.
2.3 Extraction and isolationThe dried powder of the rhizome of C. otophyllum(40 kg)was refluxed with 95% EtOH(120 L × 3). The extract was evaporated, extracted with EtOAc(6 L), and defatted with petroleum ether(1.4 L). The extract was the total glycosides(700 g). A part of the total glycosides(500 g)were dissolved in 2.25 L MeOH-0.025 mol/L H2SO4(1:2)in water bath at 70 oC. After 2 h, Ba(OH)2 solution was added to adjust the pH value to 7, and then filtered to remove BaSO4. The solution was dried up to give a crude aglycones(100 g).
The crude aglycones(100 g)were separated into 21 fractions(Frs. 1-21)through CC over silica gel(300 g)by elution with CHCl3(1874 mL), and then with a mixture of CHCl3-MeOH(100:1, 1000 mL), CHCl3-MeOH(100:3, 1000 mL), and finally CHCl3-MeOH(100:8.5, 1582 mL). Fr. 8(between 2597 and 2716 mL, 12 g)produced three fractions by CC(76 g)eluted with CHCl3-MeOH(100:1.5, 100:2, 100:3, 1000 mL each), and then the second fraction(Fr. 8-2)yielded three subfractions(Frs. 8-2-a, 8-2-b, and 8-2-c)by CC(54.5 g)eluted with petroleum ether-acetone(10:4.5, 300 mL; 10:5, 600 mL; 10:7, 227 mL), and finally the third subfraction(Fr. 8-2-c)further produced four fractions(Frs. 8-2-c-A, 8-2-c-B, 8-2-c-C, and 8-2-c-D)by CC(70.5 g)eluted with petroleum ether-ethyl acetate(35:65, 1500 mL; 0:100, 150 mL). Fr. 8-2-c-D(between 900 and 1650 mL, 0.4 g)produced two fractions by CC(40 g)eluted with petroleum ether-ethyl acetate(25:75, 500 mL). The first fraction(Fr. 8-2-c-D-1)was subjected to CC(15 g)eluted with CHCl3-MeOH(100:5, 200 mL), and one fraction(Fr. 8-2-c-D-1-1)was obtained, which was further subjected to CC(35 g)eluted with CHCl3-MeOH(100:3, 250 mL; 100:4, 10 mL; 100:10, 130 mL)to yield compound 1(between 99 and 161 mL, 89 mg, 0.089%). Fr. 14(between 4478 and 4507 mL, 0.4 g)produced two fractions by CC(74 g)eluted with CHCl3-MeOH(100:8, 1000 mL), and the first fraction(Fr. 14-1)yielded three subfractions(Frs. 14-1-1, 14-1-2, and 14-1-3)by CC(53 g)eluted with petroleum ether-acetone(10:7.5, 500 mL; 10:8, 300 mL). Subfraction 14-1-2 yielded one fraction by CC(5.5 g)eluted with CHCl3-acetone(6:4, 100 mL; 1:1, 50 mL), and this fraction(Fr. 14-1-2-1)was subjected t o CC(6 g)eluted with CHCl3-MeOH(100:8, 100 mL)to afford compound 2(between 33 and 53 mL, 46 mg, 0.046%). Fr. 8-2-c-C(between 800 and 850 mL, 30 mg)was subjected to CC(5.5 g)eluted with CHCl3-MeOH(100:4, 100 mL)(one fraction Fr. 8-2-c-C-1 was obtained), which was then subjected to CC(5.5 g)eluted with CHCl3-MeOH(100:2, 70 mL; 100:3, 20 mL), to afford compound 3(between 24 and 48 mL, 21 mg, 0.021%).
3. Results and discussionCompound 1 was obtained as a colorless gum, mp 123-126 oC, [α]20.0 D +24.6(c 0.29, EtOH). UV(EtOH)λmax(log ε): 255.6(3.21)nm; IR(KBr)υmax: 3442, 2932, 2366, 2339, 1699, 1634, 1456, 1368, 1317, 1195, 1165, 1098, 1060, 1003, 911, 537, 419 cm-1. 1H-NMR, 13C-NMR, and 2D NMR data are shown in Table 1. FAB--MS m/z: 811 [M-H]-(100), 773(4), 699(2.5), 667(13.5), 255(9.5), 85(4.5); HR-FAB--MS m/z: 811.4508 [M-H]-(calcd for C42H67O15, 811.4480). The molecular formula of compound 1 was determined as C42H68O15 by HRFAB-MS. The 13C-NMR and DEPT spectra showed one carbonyl, one pair of double bonds, nine methyls, and numerous methylenes, methines, and quaternary carbons. The spectra were compared with the 13C-NMR and DEPT data(Zhang et al, 2000)of known C21 steroidal aglycones, and the aglycone was determined to be deacetylmetaplexigenin. In compound 1, the anomeric carbon at δ 96.4 corresponded to the proton at δ 5.25 d in the HMQC spectrum, which had a long-range correlation with C-3 of the aglycone in the HMBC spectrum, and the signal for C-3 was at δ 77.9, so compound 1 was a 3-O-glycoside of deacetylmetaplexigenin. The anomeric carbon resonances at δC 96.4, 100.6, and 102.2 revealed the presence of three sugar residues. In Table 1, the proton at δ 5.25 d correlated with the signal at δ 96.4 in the HMQC spectrum, and had a correlation with H-2′ in the 1H-1H COSY spectrum. The assignment for C-2′(δ 37.0)was obtained from the correlation with H-2′(δ 1.87 m an d 2.51 m)in the HMQC spectrum. These protons had correlations with H-1′ and H-3′ in the 1H-1H COSY spectrum, from which C-3′(δ 77.8)was obtained. In this case, the carbons at δ 96.4, 37.0, 77.8, 83.2, 69.0, and 18.4 were determined to be the carbons of the sugar by 1H-1H COSY and HMQC spectra. The methoxy group(δ 58.9)was located by the correlation of its proton signal at δ 3.54 s, with C-3′ in the HMBC spectrum. The 13C-NMR data of the sugar were compared with those in literature(Zhang et al, 2000) and the sugar was determined to be α-D-ole and ropyranose. C-4′ was found at δ 83.2, and in the HMBC spectrum, it showed a long-range correlation with the proton at δ 5.10 d, which was correlated with the carbon at δ 100.6 in the HMQC spectrum. Consequently, the O-C-4′ was linked with the sugar unit whose anomeric carbon(C-1″)was at δ 100.6. On the basis of the correlations between the protons in the 1H-1H COSY spectrum and the long-range correlation of MeO- in the HMBC spectrum in Table 1, all the 13C-NMR data of the II unit were determined. The data were compared with those in literature(Zhang et al, 2000) and the II moiety was determined to be α-D-ole and ropyranose. Since H-4″(δ 3.48)had a long-range correlation with the resonance at δ 102.2 in HMBC spectrum, and C-4″ resonated at δ 83.5, so O-C-4″ was linked with the sugar unit III with the anomeric carbon at δ 102.2. This sugar was determined to be β-D-ole and ro- pyranose(Table 1). Therefore, compound 1 was elucidated as deacetylmetaplexigenin 3-O-β-D-ole and ropyranosyl-(1→4)-α-D- ole and ropyranosyl-(1→4)-α-D-ole and ropyranoside(Figure 1).
|
|
Table 1 1H-NMR and 13C-NMR spectral data for compound 1 in C5D5N(400 MHz, coupling constants in Hz) |
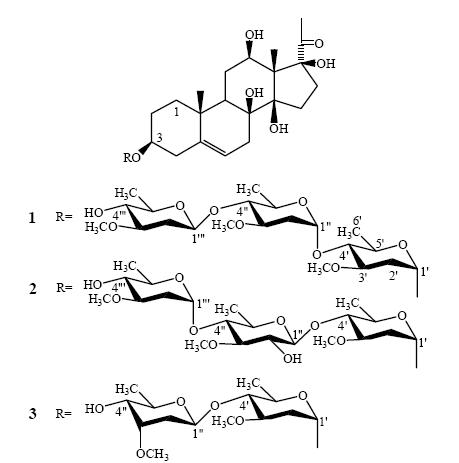 |
Figure 1 Structures of glycosides 1-3 |
Compound 2 was obtained as a colorless gum, mp 123-126 oC, [α]20.0 D +22.3(c 0.21, EtOH). UV(EtOH)λmax(log ε): 255.6(3.25)nm; IR(KBr)υmax: 3443, 2968, 2934, 1700, 1634, 1456, 1369, 1317, 1196, 1166, 1151, 1085, 1005, 912, 865, 674, 564 cm-1. 1H-NMR, 13C-NMR, and 2D NMR data are shown in Table 2. FAB--MS m/z(rel. int.): 827 [M-H]-(100), 728(1), 667(2), 515(1.5), 361(2), 283(1.5), 255(5), 99(1); HR-FAB-MS m/z: 827.4446 [M-H]-(calcd for C42H67O16, 827.4429). The molecular formula of compound 2 was determined as C42H68O16 from HRFAB-MS. The 13C-NMR and DEPT spectra were compared with those of known C21 steroidal aglycones(Zhang et al, 2000), and the aglycone unit was determined to be deacetylmetaplexigenin. In compound 2, the anomeric carbon at δ 96.4 corresponded to the proton at δ 5.25 d in HMQC spectrum, which had a long-range correlation with C-3 of the aglycone in the HMBC
spectrum, and C-3 was at δ 77.8, so this compound was also a C-3 glycoside of the deacetylmetaplexigenin. Three anomeric carbons were observed(δC 96.4, 106.3, and 100.5), revealing the presence of three sugar residues. The sugar at δ 96.4 was determined to be α-D-ole and ropyranose in the same way as previously described(Table 2). The resonance of C-4′ located at δ 83.1, and its corresponding proton(δ 3.56 m)in the HMQC spectrum, had a long-range correlation with the resonance at δ 106.3 in the HMBC spectrum. Consequently, O-C-4′ was linked with the sugar whose anomeric carbon was at δ 106.3. This sugar was determined to be β-D-theveto- pyranose(Table 2). The signal of C-4″ was at δ 83.4 whose corresponding proton(δ 3.45)in the HMQC spectrum had a long-range correlation with the carbon at δ 100.5 in the HMBC spectrum. Thus, O-C-4″ was linked with the sugar unit III. This sugar was determined to be α-D-ole and ro- pyranose(Table 2). Therefore, compound 2 was elucidated as deacetylmetaplexigenin 3-O-α-D-ole and ropyranosyl-(1→4)-β- D-thevetopyranosyl-(1→4)-α-D-ole and ropyranoside(Figure 1).
|
|
Table 2 1H-NMR and 13C-NMR spectral data for compound 2 in C5D5N(400 MHz, coupling constants in Hz) |
Compound 3 was obtained as a colorless gum, mp 101-104 oC, [α]20.0 D +42.6(c 0.20, EtOH); UV(EtOH)λmax(log ε): 255.6(2.66)nm; IR(KBr)υmax: 3442, 2932, 1700, 1634, 1456, 1367, 1318, 1274, 1194, 1165, 1147, 1089, 1062, 1004, 912, 865, 577 cm-1; 1H-NMR, 13C-NMR, and 2D NMR data are shown in Table 3; FAB--MS m/z: 667 [M - H]-(100), 559(3), 523(39.5), 280(6.5), 255(7), 194(6), 97(6.5); HR-FAB--MS m/z: 667.3668 [M - H]-(calcd for C35H55O12, 667.3694). The molecular formula of compound 3 was C35H56O12, deduced from HRFAB-MS. The 13C-NMR and DEPT spectra were compared with those of known C21 steroidal aglycones(Zhang et al, 2000), and the aglycone unit was determined to be deacetylmetaplexigenin. In compound 3, the anomeric carbon at δ 96.5 corresponded to the proton at δ 4.83 d in the HMQC spectrum, which had a long-range correlation with C-3 at δ 77.9 of the aglycone in the HMBC spectrum, and compound 3 was a 3-O-glycoside of de- acetylmetaplexigenin. The anomeric carbon resonances at δC 96.5 and 100.6 revealed the presence of two sugar residues. The sugar at δ 96.5 was determined to be α-D-ole and ropyranose in the same way as that in compound 2(Table 3). C-4′ was found to be at δ 83.7, and in the HMBC spectrum, it displayed a long-range correlation with the proton at δ 4.73, showing an HMQC correlation with the carbon at δ 100.6. This sugar was determined to be β-D-cymaropyranose(Table 3). Therefore, compound 3 was elucidated as deacetylmetaplexigenin 3-O-β-D-cymaropyranosyl-(1→4)-α- D-ole and ropyranoside(Figure 1).
|
|
Table 3 1H-NMR and 13C-NMR spectral data for compound 3 in C5D5N(400 MHz, coupling constants in Hz) |
In conclusion, C21 steroidal glycosides in Qingyangshen Tablets are composed of a C21 steroidal aglycone moiety of the 3-OH substitution and a sugar chain consisted of several sugar residues which are 2-deoxy-hexose. All of the linkages among them are 1→4 linkages. Since Qingyangshen Tablets are particularly effective against epilepsy and chronic hepatitis, this structure class is particularly effective against epilepsy and chronic hepatitis, which is the structure-activity relationship of Qingyangshen Tablets.
| [1] | Hayashi K,Mitsuhashi H,1972. Tentative structure of wilforine. Chem Pharm Bull 20(9): 2065-2067. |
| [2] | Mu QZ,Lu JR,Zhou QL,1986. Two new antiepilepsy compounds - otophyllosides A and B. Sci Sin Ser B (Engl Ed) 29(3): 295-301. |
| [3] | Mu QZ,Zhou QL,1983a. Studies on constituents of Cynanchum otophyllum Schneid roots. Acta Bot Yunnan 5(1): 99-103. |
| [4] | Mu QZ,Zhou QL,1983b. Study on chemical constituents of Qing Yang Shen (Cynanchum otophyllum Schneid.). Acta Pharm Sin 18(5): 356-362. |
| [5] | Pei YQ,Cao LG,Xie SJ,Cai ZJ,Mu QZ,1981. Central pharmacological action of Cynanchum otophyllum Schneid. J Beijing Med Univ 13(3): 213-218. |
| [6] | Pei YQ,Dai J,Chen WX,Lu ZQ,Mu QZ,1987. Central pharmacological action of three kinds of alkaloids from Cynanchum roots. J Beijing Med Univ 19(1): 29-32. |
| [7] | Zhang YH,Wen YY,Kuang TY,2000. The use of 13C NMR in the structure analysis of C21 steroidal glycosides in the Asclepiadaceae. Nat Prod Res Dev 12(3): 83-87. |
| [8] | Zhao YB,Fan QS,Xu GL, Feng ZL,Hao XJ,2008a.Isolation and structural study on carbohydrates from Cynanchum otophyllum and Cynanchum paniculatum. J Carbohyd Chem 27(7): 401-410. |
| [9] | Zhao YB,Liu D,Xu GL,Zuo GY,Zhang Q,2007. Carbohydrates from Cynanchum otophyllum. Med J Southwest Natl Def 17(4): 385-389. |
| [10] | Zhao YB,Ren HY,Zhang PH,Zuo GY,Zhang Q,Xu GL,2008b. C21 steroidal glycosides with seven sugar residues extracted from Cynanchum otophyllum Schneid. Med J Southwest Natl Def 18(5): 625-631. |
| [11] | Zhao YB,Ren HY,Zuo GY, Zhang Q,Xu GL,2009. Component analysis of extract from Cynanchum otophyllum Schneid. Med J Southwest Natl Def 19(10): 961-965. |
| [12] | Zhao YB,Shen YM,He HP,Mu QZ,Hao XJ,2005. A new C21 steroidal glycoside from Cynanchum otophyllum. Acta Bot Yunnan 27(4): 443-446. |
| [13] | Zhou J,1991. Bioactive glycosides from Chinese medicines. Mem Inst Oswaldo Cruz 86(Suppl 2): 231-234. |
 2014, Vol. 6
2014, Vol. 6


