Licorice(the roots of Glycyrrhiza uralensis Fisch., G. inflata Bat., and G. glabra L.), belonging to the Leguminosae family, is a dwarf shrub with oval leaflets, white or purplish flower clusters, flat pods, a main taproot, and numerous runners(Figure 1A). It is widespread in China, Spain, Persia, India, Afghanistan, Kazakhstan, Kyrghyzstan, Tajikistan, and Russia. North Inner Mongolia Autonomous Region, Gansu province, and Shanxi province are believed to be authentic regions of licorice in China. The Chinese name of licorice is gancao, which means “sweet grass”. In Chinese Pharmacopoeia 2010, the roots of G. uralensis, G. inflate, and G. glabra are all identified as licorice.
 | Figure 1 G. uralensis(A) and licorice slices(B) |
The roots of licorice and its slices(Figure 1B)are widely used in traditional Chinese medicine(TCM) and are honored as the reconciler in Chinese herbal compound prescriptions. The earliest written literature to the use of licorice dated from 2100 BC in Shennong’s Classic of Materia Medica, the first Chinese dispensary. In this book, licorice was recommended for its life-enhancing properties. Many modern studies have reported that licorice possesses various pharmacological activities, such as antitumor(Tao et al, 2013 ; Wang et al, 2013 ; Li et al, 2013 ), antiviral(Baltinar et al, 2012 ; Huang et al, 2012 ), anti-inflammatory(Zhang et al, 2011 ; Sun et al, 2010 ; Kim et al, 2010b ), and immunity-stimulating activities(Kim et al, 2013b ; Hong et al, 2009 ). Because of its medicinal and economic value, this Chinese herb has received considerable attention throughout the world.
In recent years, cancer has become one of the main reasons for human’s death. The conventional therapy for cancer including surgery, chemotherapy, and radiotherapy has many side effects and deficiencies. Therefore it is very important and urgent to find novel effective therapeutic approaches for treatment of cancer. Using natural compounds without side effects on human has attracted the attention of many researchers and has proved to be the single most successful strategy. Lots of researches have proved that various natural components in licorice possess effective antitumor activity. At the same time, as one of the oldest and
most frequently-used traditional herbs, it is very significant to study its pharmacological activities for the development and modernization of TCM furtherly. Therefore, in this paper, a comprehensive review about the antitumor activity of licorice is summarized. And we just hope this work can provide a basis for the intensive studies concerned with safe and effective treatment of cancer using licorice. 2. Antitumor active components
Licorice contains a lot of natural active components, including more than 20 triterpenoids such as glycyrrhizin(1) and glycyrrhetinic acid(2), and approximately 300 flavonoids such as liquiritigenin, isoliquiritigenin(8), liquiritin, isoliquiritin, and glycyrrhiza polysaccharides. Up to date, many reports have shown that compounds 1(Kim et al, 2013a ), 2(Li et al, 2012 ), prenylflavonoids(Kim et al, 2012 ), licochalcone A(3)(Kim et al, 2010a ; Orlikova et al, 2011 ; Szliszka et al, 2012 ; Kim et al, 2013c ), licochalcone B(4)(Wang et al, 2013 ), licochalcone D(5)(Wang et al, 2013 ), licochalcone E(6)(Kwon et al, 2013 ), glabridin(7), compound 8(Cuendet et al, 2010 ), dehydroglyasperin C(9), dehydroglyasperin D(10), and isoangustone A(11)(Kim et al, 2012 )possess the antitumor activity. The chemical structures of the above main components are listed in Figure 2.
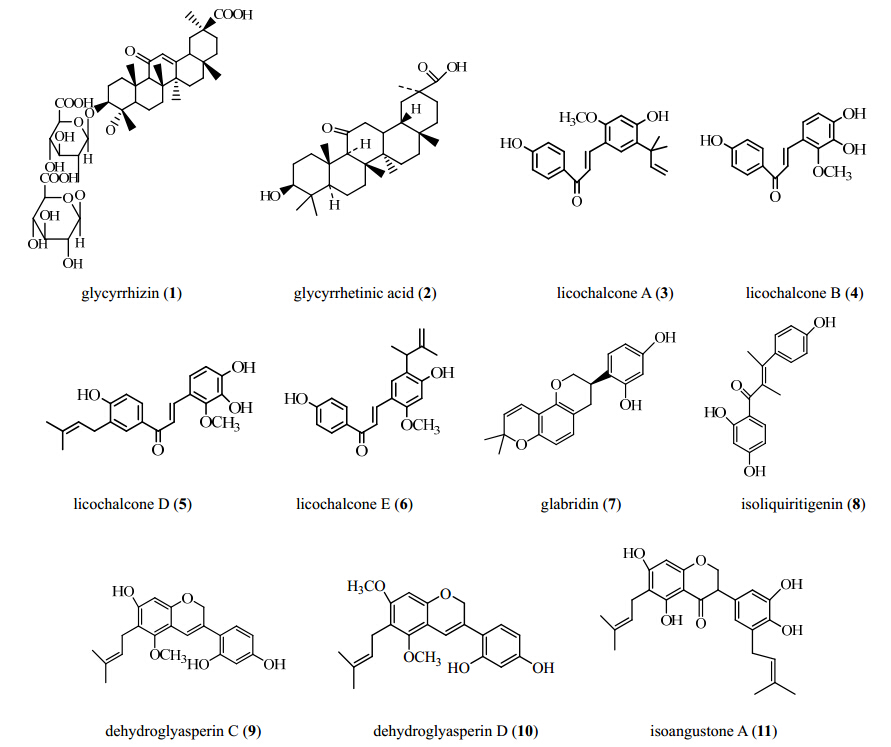 | Figure 2 Chemical structures of antitumor components in licorice |
More than 20 triterpenoids have been isolated from licorice, but only compounds 1, 2, and 11-deoxy glycyrrhetinic acid(11-DOGA)have been reported to possess the antitumor activity.
Compound 1 was believed to be the marker component in licorice for its antiviral, antitumor, and anti-inflammatory properties. Some researchers found that compound 1 had strong chemopreventive potential against DMH-induced colon carcinogenesis and it also inhibited the infiltration of mast cells(Khan et al, 2013 ). Zhao et al(2012) investigated the antitumor activity of compound 1 combined with quantum dots(QDs)in hepatocarcinoma cells. The results showed that this compound induced apoptotic response in a time- and dose-dependent manner, which suggested that it had therapeutic potential against cancer(Zhao et al, 2012 ). Compound 1 also has been reported to induce the apoptosis in leukemia cells through the caspase- and mitochondria- dependent pathways(Chueh et al, 2012 ).
Compound 2, 18α- and 18β-glycyrrhetinic acid, is the ramification of compound 1 hydrolyzed by glucuronidase. Modern pharmacological studies suggested that compound 2 was a promising anti-angiogenic therapeutic agent to target the extracellular signal-regulated kinase(ERK)pathway(Li et al, 2012 ). Kim et al(2013b) showed that compound 2 possessed a better antitumor activity and unchanged pharmacokinetic behavior. Some researchers conjugated dehydrozingerone(DZ)analogs with compound 2. In an in vitro anticancer assay using nine different human tumor cell lines, most of the conjugates showed significant potency, especially in LN-Cap, IA9, and KB cell lines. Results suggested that compound 2 was critical for antitumor activity(Tatsuzaki et al, 2007 ). Rudkin et al(2002) in his studies found that compound 2 significantly inhibited SCC-13 cell growth(P < 0.05)by altering gap junctional intercellular communication(GJIC) and the expression of connexins.
11-DOGA is produced by reduction of compound 2 11-carbonyl to 11-hydroxyl to reduce the side effects. But the reports about the pharmacological activity of 11-DOGA are very infrequent. Lin et al(2014) found that 11-DOGA obviously inhibited the viabilities of gastric cancer cells in dose- and time-dependent manners. It induced gastric cancer cell apoptosis and cell cycle arrest in G2 phase. The apoptosis in gastric cancer cells was associated with BID translocation from nucleus to mitochondria. 2.2 Flavonoids
About 300 flavonoids have been isolated from the dried licorice including phenolic acids, flavones, flavans, chalcones, isoflavonoids, etc. Up to date the main flavonoids with the glycyrrhizin(1)glycyrrhetinic acid(2)licochalcone A(3)licochalcone B(4)licochalcone D(5)licochalcone E(6)glabridin(7)isoliquiritigenin(8) dehydroglyasperin C(9)dehydroglyasperin D(10)isoangustone A(11) antitumor function in licorice are chalcones, an important polyphenolic family, including a large number of naturally occurring molecules. Polyphenolic compounds such as compounds 3-6 and 8 have shown to interfere with initiation, promotion, and progression of carcinogenesis, suggesting that they can be used as potential anticancer drugs.(Cuendet et al, 2010; Kim et al, 2010a , Orlikova et al, 2011 ; Szliszka et al, 2012 ; Kim et al, 2013c ; Wang et al, 2013 ; Kwon et al, 2013 ).
Kanazawa et al(2003) investigated the antitumor activity of compound 8 on DU145 and LNCaP prostate cancer cell lines in vitro. They found that compound 8 significantly inhibited the proliferation of DU145 and LNCaP cell lines in a dose-dependent and time-dependent manner. They also found that compound 8 enhanced the expression of GADD153 mRNA and protein associated with S and G2/M cell cycle arrest. These results suggest that compound 8 is a c and idate agent for the treatment of prostate cancer, and in which GADD153 may play an important role. Jung et al(2006) indicated that compound 8 induced apoptosis by depolarizing mitochondrial membranes in prostate cancer cells, and they cultured MAT-LyLu(MLL)rats and DU145 human prostate cancer cells with various concentration of compound 8 and found that compound 8 inhibited prostate cancer cell growth by the induction of apoptosis, which could be mediated through an evident disruption of the mitochondrial membrane potential, release of cytochrome c and Smac/Diablo, and activation of caspase-9. Park et al(2009) attempted to investigate the underlying mechanism by which compound 8 induced cell cycle arrest and cytotoxicity in HeLa human cervical cancer cells. Results showed that compound 8 functioned as a topoisomerase II poison and arrest in mitotic metaphase-like stage contributed to its antiproliferative effects. All above suggest that compound 8 is one of the antitumor components in licorice.
Kim et al(2010a) investigated the inhibition of compound 3 on carcinogenesis and metastasis in mouse models. The results showed that compound 3 significantly increased the survival of mouse and inhibited liver metastasis, which suggested that compound 3 had potent antitumor and antimetastatic activity. Szliszka et al(2012) indicated that tumor necrosis factor-related apoptosis-inducing lig and (TRAIL)induced the apoptosis in cancer cells without toxicity to normal cells, which played a very important role in immune surveillance and defense against cancer cells. Chalcones can sensitize cancer cells to TRAIL-induced apoptosis, they augmented the antitumor activity of TRAIL and confirmed their cancer chemopreventive properties. Wang et al(2013) also reported the antitumor activity of compounds 4 and 5. Yuan et al(2014) reported that exposure of human malignant bladder cancer cell lines T24 or EJ to compound 4 obviously inhibited the cell proliferation and resulted in S phase arrest in T24 or EJ cell lines. Kwon et al(2013) investigated the inhibition of compound 6 on mammary tumor growth and metastasis using animal and cell models. They found that compound 6 inhibited tumor growth and lung metastasis in the mouse model, and it also inhibited cell migration, invasion, and tube formation in vitro.
In addition to chalcones, prenylflavonoids including compounds 9-11 also showed the strong ferric reducing activities and high free radical scavenging capacity in human hepatoma HepG2 cells, which suggests that it has the potential as antitumor drugs(Kim et al, 2012 ). Compound 7 showed the effect to inhibit the migration, invasion, and angiogenesis of human non-small cell lung cancer A549 cells and MDA-MB-231 human breast adenocarcinoma cells by suppressing the focal adhesion kinase(FAK)/Rho signaling pathway(Tsai et al, 2011 ; Hsu et al, 2011 ). 2.3 Extract from licorice
Some researchers investigated the antitumor activity of licorice extract. Sheela et al(2006) identified an aqueous extract from licorice and found that it inhibited the proliferation of Ehrlich ascites tumor cells in vivo and in vitro. The extract decreased cytokine VEGF production and neovascularization. It is known that bold vessel plays an important role in solid tumor development and blocking of angiogenesis, and the action of cytokine VEGF is possible in cancer therapy. Therefore the above findings suggest that the extract from licorice may be a potential supplemental source for cancer therapy.
Rafi et al(2002) assessed licorice extract for the effects on anti-apoptotic protein Bcl-2 to identify novel cytotoxic derivatives. The results showed that Bcl-2 phosphorylation and G2/M cell cycle arrest were induced by licorice extract, which was similarly to clinically used antimicrotubule agents such as paclitaxel. Six compounds were identified in the following HPLC separation experiment, and one of them was responsible for Bcl-2 phosphorylation. The compound was identified as 1-(2, 4-dihydroxyphenyl)-3-hydroxyl-3-(4′- hydroxyphenyl)-1-propanone(β-hydroxy-DHP). They believed that the effect on Bcl-2 was structure specific, and Bcl-2 phosphorylation, G2/M cell cycle arrest, apoptosis, and altered microtubule structure in breast and prostate tumor cells were induced by pure β-hydroxy-DHP.
Lee et al(2013) and Seon et al(2012) also reported that the ethanol extract and the hexane/ethanol extract of roasted licorice had the anti-breast cancer activity by inhibiting cell-cycle progression in DU145 human prostate and 4T1 mouse breast cancer cells. 3. Antitumor activity and possible mechanism for cancer prevention
Up to date, the licorice extract including all above components has shown the significant inhibitory effects on colorectal cancer(Leonetti et al, 2006 ; Huang et al, 2014 ), breast cancer(Tatsuzaki et al, 2007 ; Rossi et al, 2003 ; Li et al, 2013 ; Lorusso and Marech, 2013 ; Lee et al, 2013 ; Kwon et al, 2013 ; Wang et al, 2013a ; 2013b; Seon et al, 2012 ; Patel et al, 2011 ), prostate cancer(Rafi et al, 2002 ; Kanazawa et al, 2003 ; Jung et al, 2006 ; Shetty et al, 2011 ; Lee et al, 2013 ; Szliszka et al, 2010 ), glioblastoma(Li et al, 2014 ), etc. Among ascites tumor(Sheela et al, 2006 ), liver cancer(Kim et al, 2008 ; Zhang et al, 2012 ; Zhao et al, 2012 ; Wei et al, 2011 ; Tsai et al, 2014 ), gastric cancer(Xiao et al, 2011 ; Lin et al, 2014 ; Lee et al, 2011 ), uterus tumor(Park et al, 2009 ), melanoma(Song et al, 2013 ), leukemia(Chueh et al, 2012 ), bladder cancer(Jiang et al, 2014 ; Yuan et al, 2014 ), lung cancer(Tsai et al, 2011 ; Seo, 2013 ; Choi et al, 2013 ), oral cancer(Kim et al, 2014 ; Lee et al, 2012 ), and a variety of solid tumors(Yoon et al, 2007 ; Lee et al, 2008 ; Jung et al, 2006 ). When combined with chemotherapy drugs cisplatin, it has also shown an activity to reduce the oxidative stress and decrease its side effects. 3.1 Colorectal cancer
Khan et al(2013) indicated that compound1 obviously attenuated the level of TNF-α and it also declined the depletion of the mucous layer and the shifting of sialomucin to sulphomucin. Their findings suggested that compound 1 had strong chemopreventive potential against DMH-induced colon carcinogenesis(Khan et al, 2013 ). Huang et al(2014) tested the antitumor effect of a group of representative licorice-derived compounds and found that compound 11 promptly inhibited the survival of SW480 human colorectal cancer cells by inducing mitochondrial outer membrane in a time- and concentration-dependent pattern. It activated capase-dependent pro-apoptotic signaling and induced significant apoptosis. It strongly inhibited Akt phospho- rylation in 5 min. It deserves further investigations as a novel anti-colorectal cancer agent(Huang et al, 2014 ). 3.2 Breast cancer
In all of the reports about antitumor activity of licorice, the reports about anti-breast cancer are the most. In the past three years, many researchers contributed to this field. Lorusso and Marech(2013) found that low concentration of compound 8 had therapeutic potential in the treatment of aggressive breast cancer. Lee et al(2013) reported that the ethanol extract of fried licorice inhibited breast cancer- mediated bone destruction. Kwon et al(2013) suggested that compound 6 directly inhibited the migration and invasion of both MDA-MB-231 human breast cancer cells and 4T1 cells. Wang et al(2013) suggested that compound 8 also suppressed the migration of MDA-MB-231 cells by inhibiting the upstream signaling pathways, this conclusion was according to Wang et al(2013) . Seon et al(2012) found the anti-breast cancer activity of hexane/ethanol extract from G. uralensis by inhibiting cell-cycle progression in DU145 human prostate and 4T1 mouse breast cancer cells. Hsu et al(2011) indicated that compound 7 inhibited migration, invasion, and angiogenesis of MDA-MB-231 human breast adenocarcinoma cells by inhibiting focal adhesion kinase/Rho signaling pathway. 3.3 Prostate cancer
Shetty et al(2011) investigated the anticancer effects and mechanism of compound 2 on the and rogen-independent metastatic prostate cancer cell line DU-145 and found that compound 2 inhibited the proliferation and growth of DU-145 cells by inducing apoptosis and prevented the invasion of DU-145 cells on matrigel coated transwells by down-regulation of NF-κB, VEGF, and NMP-9 expression. This study suggests that compound 2 may be a promising anticancer agent for the chemoprevention and treatment of prostate cancer. Lee et al(2013) analyzed the inhibitory effects of compound 11 on the growth of PTEN-deleted human prostate cancer cells in vitro and in vivo. They found that compound 11 was a potent molecular inhibitor of CDK2 and mammalian target of rapamycin(mTOR)for the treatment of prostate cancer. Szliszka et al(2010) examined the cytotoxic and apoptotic effects of chalcones and dihydrochalcones on TRAIL-mediated apoptosis in LNCaP prostate cancer cells and confirmed the important role of chalcones in chemoprevention of prostate cancer. 3.4 Liver cancer
The inhibiting effect of compound 1 on the proliferation of liver cancer cell SMMC-7721 was investigated in vitro(Zhang et al, 2012 ). The results showed that compound1 inhibited the growth of SMMC-7221 obviously, and the mechanism was related to the up-regulation of p53 expression. Tsai et al(2014) reported that compound 3 inhibited the migratory and invasive abilities of human hepatocellular carcinoma cell SK-Hep-1 and HA22T/VGH in a dose-dependent manner. It was found that compound 3 induced a dose-dependent inhibition of uPA activity and expression, as well as reduced mRNA levels in SK-Hep-1 and HA22T/VGH cells. It was also found to inhibit the expression of phosphor-JNK and phosphor-MKK4 in SK-Hep-1 cells. 3.5 Gastric cancer
Xiao et al(2011) investigated the anticancer effects of seven licorice compounds in MKN-28, MKN-45, and AGS gastric cancer cells and human gastric epithelium immortalized cells. The results showed that compound 3 was the most cytotoxic licorice compound of the seven compounds, and it inhibited gastric cancer cells growth in a dose-dependent manner by blocking cell cycle progression at the G2/M transition and inducing apoptosis. Lin et al(2014) reported that compound 2 and 11-DOGA effectively inhibited tumor formation of gastric cancer cells in nude mice, they induced gastric cancer cells apoptosis and cell cycle arrest in G2 phase. 3.6 Bladder cancer
The reports about anti-bladder cancer effects of licorice are relatively infrequent. In the past three years, only compounds 3 and 4 have been reported to inhibit the proliferation of bladder cancer. Researches of Jiang et al(2014) indicated that compound 3 inhibited human bladder cancer T24 proliferation by increasing the levels of intracellular ROS, and resulted in an oxidative stress status in T24 cells, with the IC50 value of approximately 55 μmol/L. Yuan et al(2014) investigated the mechanisms by which compound 4 inhibited the proliferation of human bladder cancer cell lines(T24 and EJ)in vitro and antitumor activity in vivo in MB49 tumor model. Results showed that compound 4 significantly inhibited T24 or EJ cell lines proliferation in a concentration- and time-dependent manner. Consistently, compound 4 also significantly limited the tumorigenicity of MB49 cells. These reports provided support for the use of compound 4 in chemoprevention and bladder cancer therapy. 3.7 Lung cancer
Tsai et al(2011) reported that compound 7 inhibited the migration and invasion of human non-small cell lung cancer A549 cells, and it also decreased A549-mediated angiogenesis by suppressing the FAK/Rho signaling pathway. Compound 7 decreased the active forms of FAK and Src, and enhanced the levels of inactivated phosphorylated Src. The interaction of FAK and Src was decreased, which also blocked Akt activation, resulting in reduced activation of RhoA and myosin light chain phosphorylation. Therefore compound 7 may be a novel anticancer agent for the therapy of lung cancer in three different ways such as inhibition of migration, invasion, and angiogenesis. Choi et al(2013) reported that compound 3 induced the apoptosis in Hep G2 human hepato- cellular carcinoma cells by endoplasmic reticulum stress via a phospholipase Cγ1-, Ca2+-, and reactive oxygen species(ROS)-dependent pathway. 3.8 Other cancers
The reports about antitumor effects of licorice on the oral cancer, human melanoma, and leukemia are relatively infrequent. The findings of Kim et al(2014) demonstrated that compound 3 induced the apoptosis in KB oral cancer cells by a caspase-dependent FasL-mediated death receptor pathway. Song et al(2013) found that compound 11 inhibited the proliferation of human melanoma cells. It obviously suppressed the cell-cycle progression at G1 phase and blocked the expression of G1 phase regulatory proteins, including cyclins D1 and E in SK-MEL-28 human melanoma cell line. Chueh et al(2012) reported that compound 1 induced the apoptosis in leukemia cells. And the possible mechanism of this effect is through the caspase- and mitochondria- dependent pathways.
Based on the above, the licorice extract and many single components including triterpenes such as compounds 1, 2, and 11-DOGA, and flavonoids such as compounds 3, 4, and 8, have shown a significant inhibitory effect on dozens of cancers. Among them, flavonoids, especially chalcones as an important polyphenolic family, play a very important role. The antitumor active components exert their cytotoxic activity multitudinously by increasing the intracellular ROS levels, enhancing the mRNA expression of p53 and GADD153, and inducing the apoptosis and mitochondrial outer membrane. They also inhibit the upstream and focal adhesion kinase/Rho signaling pathway, the activity of topoisomerase II, Ca2+, protein kinase C(PKC), c-Jun N-terminal Kinase 1, the expression of NF-κB, VEGF, NMP-9, uPA, G1 phase regulatory proteins, and the mitochondrial membrane potential. They reduce the depletion of the mucous layer, the shifting of sialomucin to sulphomucin, and the cancer cell proliferation. 4. Discussion
The licorice is widely used in TCM. The dem and for this herb is very huge in China. Recent irresponsible excessive exploitation of wild licorice has caused the decrease and extinction of wild licorice resources. The Chinese government has imposed restrictions on the collection of wild licorice plants. A systematic study of pharmacological activities is helpful for the effective use and conservation of licorice. In this paper, one of the pharmacological activities of licorice, the antitumor activity, is summarized in detail.
A large and growing body of evidence has shown that licorice may be an effective herbal medicine for chemoprevention. Many single components including triterpenes and flavonoids have shown a significant inhibitory effect on dozens of cancers. It is time to develop a human clinical trial under carefully controlled conditions with a high-risk population. Many researches also showed that the biological activities of licorice are due to the effects of several licorice components combination, and the licorice extract seemed more effective than a specific active ingredient on some diseases. And we suggest this is an interesting direction of research.
In some previous reports, the clinical safety of compounds 1 and 2 has been evaluated(Shibata et al, 2000 ; Olukoga and Donaldson, 2000 ; Vogel et al, 1992 ). It is true that some people are sensitive to compounds 1 or 2, but when patients with previous breast cancer received 0.02-0.04 mmol/kg(body weight)without evidence of significant toxicity, there was apparently a great individual variation in the susceptibility to compound 2. It is necessary to develop a special strategy to evaluate the possible adverse effects of licorice triterpenes. The evaluations about other components of licorice are very infrequent. The antitumor activity of flavonoid, especially chalcone is deserved to confirm. Looking for new triterpenes with a skeleton similar to compound 1 without side-effects is another very interesting future direction of research.
The combination or prescription of several drugs on the basis of their synergistic activity is a new strategy for modern cancer prevention. In TCM, using a single herb is very rare for disease treatment. So the combination of licorice and other herbs possibly has a significant effect on cancer treatment.
This paper indicates that licorice represents interesting and important hits for antitumor drug discovery and development. And we just hope this work can provide a basis for further studies concerned with fully revealing the antitumor mechanism of licorice and safe use of licorice.
| [1] | Baltinar LA,Chistoedova ES,Baltina LA,Kondratenko RM,Plyasunova OA,2012. Synthesis and anti-HIV-1 activity of new conjugates of 18β- and 18α-glycyrrhizic acids with aspartic acid esters. Chem Nat Compd 48(2): 262-266 . |
| [2] | Choi AY,Choi JH,Hwang KY,Jeong YJ,Choe W,Yoon KS,Ha J,Kim SS,Youn JH,Yeo EJ,Kang I,2013. Licochalcone A induces apoptosis through endoplasmic reticulum stress via a phospholipase Cγ1-,Ca2+-,and reactive oxygen species- dependent pathway in HepG2 human hepatocellular carcinoma cells. Apoptosis 19: 682-697 . |
| [3] | Chueh FS,HsiaoYT,Chang SJ,Wu PP,Yang JS,Lin JJ,Chung JG,Lai TY,2012. Glycyrrhizic acid induces apoptosis in WEHI-3 mouse leukemia cells through the caspase-and mitochondria- dependent pathways. Oncol Rep 28(6): 2069-2076 . |
| [4] | Cuendet M,Guo J,LuoY,Chen S,Oteham CP,Moon RC,Pezzuto JM,2010. Cancer chemopreventive activity and metabolism of isoliquiritigenin,a compound found in licorice. Cancer Prev Res 3(2): 221-232 . |
| [5] | Hong YK,Wu HT,Ma T,Liu WJ,He XJ,2009. Effects of Glycyrrhiza glabra polysaccharides on immune and antioxidant activities in high-fat mice. Int J Biol Macromol 45(1): 61-64 . |
| [6] | Hsu YL,Wu LY,Hou MF,Tsai EM,Lee JN,Liang HL,Jong YJ,Hung CH,Kuo PL,2011. Glabridin,an isoflavan from licorice root,inhibits migration,invasion and angiogenesis of MDA-MB-231 human breast adenocarcinoma cells by inhibiting focal adhesion kinase/Rho signaling pathway. Mol Nutr Food Res 55(2): 318-327 . |
| [7] | Huang W,Chen X,Li Q,Li P,Zhao GN,Xu MM,Xie P,2012. Inhibition of intercellular adhesion in herpex simplex virus infection by glycyrrhizin. Cell Biochem Biophys 62(1): 137-140 . |
| [8] | Huang W,Tang S,Qiao X,Ma W,Ji S,Wang K,Ye M,Yu S,2014. Isoangustone A induces apoptosis in SW480 human colorectal adenocarcinoma cells by disrupting mitochondrial functions. Fitoterapia 94: 36-47 . |
| [9] | Jiang J,Yuan X,Zhao H,Yan X,Sun X,Zheng Q,2014. Licochalcone A inhibiting proliferation of bladder cancer T24 cells by inducing reactive oxygen species production. Biol Med Mater Eng 24(1): 1019-1025 . |
| [10] | Jung JI,Lim SS,Choi HJ,Cho HJ,Shin HK,Kim EJ,Chung WY,Park KK,Park JHY,2006. Isoliquiritigenin induces apoptosis by depolarizing mitochondrial membranes in prostate cancer cells. J Nutr Biochem 17(10): 689-696 . |
| [11] | Kanazawa M,Satomi Y,Mizutani Y,Ukimura O,Kawauchi A,Sakai T,Baba M,Okuyama T,Nishino H,Miki T,2003. Isoliquiritigenin inhibits the growth of prostate cancer. Eur Urol 43(5): 580-586 . |
| [12] | Khan R,Khan AQ,Lateef A,Muneeb U,Rehman,TahirM,AliF,Oday O,Hamiza,Sultana S,2013. Glycyrrhizic acid suppresses the development of precancerouslesions via regulating the hyperproliferation,inflammation,angiogenesis and apoptosis in the colon of Wistar rats. PLoS One 8(2): e56020 . |
| [13] | Kim HJ,Seo JY,Suh HJ,Lim SS,Kim JS,2012. Antioxidant activities of licorice-derived prenylflavonoids. Nutr Res Pract 6(6): 491-498 . |
| [14] | Kim JK,Shin EK,Park JH,Kim YH,Park JHY,2010a. Antitumor and antimetastatic effects of licochalcone A in mouse models. J Mol Med 88(8): 829-838 . |
| [15] | Kim JS,Park MR,Lee SY,Kim DK,Moon SM,Kim CS,Cho SS, Yoon G,Im HJ,You JS,Oh JS,Kim SG,2014. Licochalcone A induces apoptosis in KB human oral cancer cells via a caspase-dependent FasL signaling pathway. Oncol Rep 31(2): 755-762. |
| [16] | Kim KJ,Choi JS,Kim KW,Jeong JW,2013a. The anti-angiogenic activities of glycyrrhizic acid in tumor progression. Phytother Res 27(6): 841-846 . |
| [17] | Kim ME,Kim HK,Kim DH,Yoon JH,Lee JS,2013b. 18β-Glycyrrhetinic acid from licorice root impairs dendritic cells maturation and Th1 immune responses. Immunopharm Immunot 35(3): 329-335 . |
| [18] | Kim SC,Park SJ,Lee JR,Seo JC,Yang CH,Byun SH,2008. Cytoprotective activity of Glycyrrhizae Radix extract against arsenite-induced cytotoxicity. Evid-Based Compl Alt 5(2): 165-171 . |
| [19] | Kim YH,Shin EK,Kim DH,Lee HH,Park JHY,Kim JK,2010b. Antiangiogenic effect of licochalcone A. Biochem Pharmacol 80(8): 1152-1159 . |
| [20] | Kim YJ,Jung EB,Myung SC,Kim W,Lee CS,2013c. Licochalcone A enhances geldanamycin-induced apoptosis through reactive oxygen species-mediated caspase activation. Pharmacology 92(1/2): 49-59 . |
| [21] | Kwon SJ,Park SY,Kwon GT,Lee KW,Kang YH,Choi MS,Yun JW,Jeon JH,Jun JG,Park JHY,2013. Licochalcone E present in licorice suppresses lung metastasis in the 4T1 mammary orthotopic cancer model. Cancer Prev Res 6: 603-613 . |
| [22] | Lee CS,Kwak SW,Kim YJ,Lee SA,Park ES,Myung SC,Kim W,Lee MS,Lee JJ,2012. Guanylate cyclase activator YC-1 potentiates apoptotic effect of licochalcone A on human epithelial ovarian carcinoma cells via activation of death receptor and mitochondrial pathways. Eur J Pharmacol 683(1): 54-62. |
| [23] | Lee DH,Park KI,Park HS,Kang SR,Nagappan A,Kim JA,Kim EH,Lee WS,Hah YS,Chung HJ,An SJ,Kim GS,2012. Flavonoids isolated from Korea Citrus aurantium L. induce G2/M phase arrest and apoptosis in human gastric cancer AGS cells. Evid-Based Compl Alt: 515901-515911. |
| [24] | Lee E,Son JE,Byun S,Lee SJ,Kim YA,Liu K,Kim J,Lim SS,Park JH,Dong Z,Lee KW,Lee HJ,2013. CDK2 and mTOR are direct molecular targets of isoangustone A in the suppression of human prostate cancer cell growth. Toxicol Appl Pharmacol 272(1): 12-20 . |
| [25] | Lee SG,Oh HM,Lim WB,Choi EJ,Park YN,Kim JA,Choi JY,Hong SJ,Oh HK,Son JK,Lee SH,Kim OJ,Choi HR,Jun CD,2008. Gene induction by glycyrol to apoptosis through endonuclease G in tumor cells and prediction of oncogene function by microarray analysis. Anti-cancer Drugs 19(5): 503-515 . |
| [26] | Lee SK,Park KK,Park JH,Lim SS,Chung WY,2013. The inhibitory effect of roasted licorice extract on human metastatic breast cancer cell-induced bone destruction. Phytother Res 27(12): 1776-1783 . |
| [27] | Leonetti C,Scarsella M,Zupi G,Zoli W,Amadori D,Medri L,Fabbri F,Rosetti M,Ulivi P,Cecconetto L,Bolla M,Tesei A,2006. Efficacy of a nitric oxide–releasing nonsteroidal anti- inflammatory drug and cytotoxic drugs in human colon cancer cell lines in vitro and xenografts. Mol Cancer Ther 5(4): 919-926 . |
| [28] | Li J,Xu H,Ke X,Tian J,2012. The anti-tumor performance of docetaxel liposomes surface-modified with glycyrrhetinic acid. J Drug Target 20(5): 467-473 . |
| [29] | Li S,Zhu JH,Cao LP,Sun Q,Liu HD,Li WD,Li JS,Hang CH,2014. Growth inhibitory in vitro effects of glycyrrhizic acid in U251 glioblastoma cell line. Neurol Sci doi: 10.1007/s10072-014- 1661-4 (ahead-of-print) |
| [30] | Li Y,Zhao HX,Wang YZ,Zheng H,Yu W,Chai HY,Zhang J,Falck JR,Guo AM,Yue J,Peng RX,Yang J,2013. Isoliquiritigenin induces growth inhibition and apoptosis through downregulating arachidonic acid metabolic network and the deactivation of P13K/Akt in human breast cancer. Toxicol Appl Pharm 272: 37-48 . |
| [31] | Lin D,Zhong W,Li J,Zhang B,Song G,Hu T,2014. Involvement of BID translocation in glycyrrhetinic acid and 11-Deoxy glycyrrhetinic acid-induced attenuation of gastric cancer growth. Nutr Cancer doi: 10.1080/01635581.2013.877498 (ahead-of-print). |
| [32] | Lorusso V,Marech I,2013. Novel plant-derived target drugs: A step forward from licorice? Expert Opin Ther Targets 17(4): 333-335. |
| [33] | Olukoga A,Donaldson D,2000. Liquorice and its health implications. J R Soc Health 120: 83-89 . |
| [34] | Orlikova B,Tasdemir D,Golais F,Dicato M,Diederich M,2011. Dietary chalcones with chemopreventive and chemotherapeutic potential. Genes Nutr 6(2): 125-147 . |
| [35] | Park I,Park KK,Park JHY,Chung WY,2009. Isoliquiritigenin induces G2 and M phase arrest by inducing DNA damage and by inhibiting the metaphase/anaphase transition. Cancer Lett 277(2): 174-181 . |
| [36] | Patel K,Karthikeyan C,Raja SV,S. Hari NMN,Lee H,Sahu K,Singh DG,Trivedi P,2011. Synthesis of some coumarinyl chalcones and their antiproliferative activity against breast cancer cell lines. Lett Drug Des Discov 8(4): 308-311. |
| [37] | Rafi MM,Vastano BC,Zhu N,Ho CT,Ghai G,Rosen RT,Gallo MA,DiPaola RS,2002. Novel polyphenol molecule isolated from licorice root (Glycrrhiza glabra) induces apoptosis,G2/M cell cycle arrest,and Bcl-2 phosphorylation in tumor cell lines. J Agr Food Chem 50(4): 677-684 . |
| [38] | Rossi T,Castelli M,Zandomeneghi G,2003. Selectivity of action of glycyrrhizin derivatives on the growth of MCF27 and HEP2 cells. Anticancer Res 23(5A): 3813-3818. |
| [39] | Rudkin GH,Carlsen BT,Chung CY,Huang WB,Ishida K,Anvar B,Yamaguchi DT,Miller TA,2002a. Retinoids inhibit squamous cell carcinoma growth and intercellular communication. J Surg Res 103(2): 183-189 . |
| [40] | Seo YH,2013. Discovery of licochalcone A as a natural product inhibitor of Hsp90 and its effect on gefitinib resistance in non-small cell lung cancer (NSCLC). Notes 34(6): 1917. |
| [41] | Seon MR,Park SY,Kwon SJ,Lim SS,Choi HJ,Park H,Park JHY. 2012. Hexane/ethanol extract of Glycyrrhiza uralensis and its active compound isoangustone A induce G1 cycle arrest in DU145 human prostate and 4T1 murine mammary cancer cells. J Nutr Biochem 23(1): 85-92. |
| [42] | Sheela ML,Ramakrishna MK,Salimath BP,2006. Angiogenic and proliferative effects of the cytokine VEGF in Ehrlich ascites tumor cells is inhibited by Glycyrrhiza glabra. Int Immunopharmacol 6(3): 494-498 . |
| [43] | Shetty AV,Thirugnanam S,Dakshinamoorthy G,Samykutty A,Zheng G,Chen A,Bosland MC,Kajdacsy-Balla A,Gnanasekar M,2011. 18α-glycyrrhetinic acid targets prostate cancer cells by down-regulating inflammation-related genes. Int J Oncol 39(3): 635-640. |
| [44] | Shibata S,2000. A drug over the millennia: Pharmacognosy,chemistry,and pharmacology of licorice. Yakugaku Zasshi 120: 849-864. |
| [45] | Song NR,Lee E,Byun S,Kim JE,Mottamal M,Park JHY,Lim SS,Bode AM,Lee HJ,Lee KW,Dong Z,2013. Isoangustone A,a novel licorice compound,inhibits cell proliferation by targeting P13K,MKK4 and MKK7 in human melanoma. Cancer Prev Res 6(12): 1293-1303 . |
| [46] | Sun ZJ,Chen G,Zhang W,Hu X,Huang CF,Wang YF,Jia J,Zhao YF,2010. Mammalian target of rapamycin pathway promotes tumor-induced angiogenesis in adenoid cystic carcinoma: Its suppression by isoliquiritigenin through dual activation of c-Jun NH2-terminal kinase and inhibition of extracellular signal-regulated kinase. J Pharmacol Exp Ther 334(2): 500-512 . |
| [47] | Szliszka E,Czuba ZP,Mazur B,Paradysz A,Krol W,2010. Chalcones and dihydrochalcones augment TRAIL-mediated apoptosis in prostate cancer cells. Molecules 15(8): 5336-5353 . |
| [48] | Szliszka E,Jaworska D,Ksek M,Czuba ZP,Król W,2012. Targeting death receptor TRAIL-R2 by chalcones for TRAIL-induced apoptosis in cancer cells. Int J Mol Sci 13(11): 15343-15359. |
| [49] | Tao WW,Du JA,Yang NY,Li JP,Tang YP,Yan H,2013. Chemical constituents of triterpenoid saponins from Glycyrrhiza uralensis. Chin Tradit Herb Drugs 44(12): 1552-1557. |
| [50] | Tatsuzaki J,Taniguchi M,Bastow KF,Nakagawa-Goto K,Morris-Natschke SL,Itokawa H,Baba K,Lee KH,2007. Anti- tumor agents 255: Novel glycyrrhetinic acid-dehydrozingerone conjugates as cytotoxic agents. Bioorgan Med Chem 15(18): 6193-6199 . |
| [51] | Tsai JP,Hsiao PC,Yang SF,Hsieh SC,Bau DT,Ling CL,Pai CL,Hsieh YH,2014. Licochalcone A suppresses migration and invasion of human hepatocellular carcinoma cells through downregulation of MKK4/JNK via NF-κB mediated urokinase plasminogen activator expression. PLoS One 9(1): e86537 . |
| [52] | Tsai YM,Yang CJ,Hsu YL,Wu LY,Tsai YC,Hung JY,Lien CT,Huang MS,Kuo PL,2011. Glabridin inhibits migration,invasion,and angiogenesis of human non–small cell lung cancer A549 cells by inhibiting the FAK/Rho signaling pathway. Integr Cancer Ther 10(4): 341-349 . |
| [53] | Vogel VG,Newman RA,Ainslie N,Winn RJ,1992. Phase I pharmacology and toxicity study of glycyrrhetinic acid as a chemopreventive drug (abstr). Proc Am Assoc Cancer Res 33: 208. |
| [54] | Wang KL,Hsia SM,Chan CJ,Chang FY,Huang CY,Bau DT,Wang PS,2013. Inhibitory effects of isoliquiritigenin on the migration and invasion of human breast cancer cells. Expert Opin Ther Targets 17(4): 337-349 . |
| [55] | Wang Z,Liu Z,Cao Y,Paudel S,Yoon G,Cheon SH. 2013. Short and efficient synthesis of licochalcone B and D through acid-mediated claisen-schmidt condensation. Notes 34(12): 3907. |
| [56] | Wei S,2011. Effect of magnesium isoglycyrrhizinate and tiopronin on preventing hepatic injury induced by chemotherapy in breast cancer. J Clin Med Practice 15 (9): 73-75. |
| [57] | Xiao XY,Hao M,Yang XY,Ba Q,Li M,Ni SJ,Wang LS,Du X,2011. Licochalcone A inhibits growth of gastric cancer cells by arresting cell cycle progression and inducing apoptosis. Cancer Lett 302(1):69-75 . |
| [58] | Yao K,Chen HY,Lee MH,Li HT,Ma WY,Peng C,Song NR,Lee KW,Bode AM,Dong ZM,Dong ZG,2014. Licochalcone A,a natural inhibitor of c-Jun N-terminal kinase 1. Cancer Prev Res 7(1): 139-149 . |
| [59] | Yoon G,Kang BY,Cheon SH,2007. Topoisomerase inhibition and cytotoxicity of licochalcones A and E from Glycyrrhiza inflate. Arch Pharm Res 30(3): 313-316 . |
| [60] | Yuan X,Li T,Xiao E,Zhao H,Li Y,Fu S,Wang Z,2014. Licochalcone B inhibits growth of bladder cancer cells by arresting cell cycle progression and inducing apoptosis. Food Chem Toxicol 65: 242-251 . |
| [61] | Zhang JF,Li H,Li CQ,Zhao CL,Tang HM,Yan RG,2012. Effect of diammonium glycyrrhizinate on proliferation in liver cancer cell SMMC-7721 and p53 expression. Chongqing Med 41 (27): 2852-2856 . |
| [62] | Zhao MX,Ji LN,Mao ZW,2012. β-Cyclodextrin/glycyrrhizic acid functionalised quantum dots selectively enter hepatic cells and induce apoptosis. Chem Eur J 18(6): 1650-1658 . |
 2014, Vol. 6
2014, Vol. 6


