2. Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing100193, China
3. Palm Biologicals, LLC. Albany, USA
4. Center for Biotechnology & Interdisciplinary Studies, Rensselaer Polytechnic Institute, Troy, USA
5. Center for Immunology and Microbial Disease, Albany Medical College, Albany, USA
6. Translational Medicine, Albany Medical College, Albany, USA
7. Department of Chemistry, State University of New York at Albany, Albany, USA
Traditional Chinese medicine(TCM)has rich history in disease theory and treatment practices that have been established in China dating back more than 2000 years. TCM principles are rooted in books such as Yellow Emperor’s Inner Canon compiled around the first century BC, and Treatise on Cold Damage dating back to 196 AD(TCM, 2009; Kaptchuk, 2000; Wiseman, 1996; McNamara, 2012). Compendium of Materia Medica is the first written compilation of such practices published in 1578 by Shi-zhen Li, and is regarded as the most complete compilation of various TCM practices(Chen et al, 2012), which has been translated into more than 20 languages, and is in print today, used as a reference manual by TCM practitioners. Common TCM practices include herbal treatments, diet, acupuncture, massage, exercise, etc. TCM uses a complex system-based disease model that categorizes the human body into functional entities. Illnesses resulting from disharmony or imbalance patterns within the individual provide the basis for treating with specific TCMs, including Chinese herbal medicines(CHM)that focus on ameliorating disease or symptoms(Kaptchuk, 2000; O'Brien, 2003; Wiseman, 1996; McNamara, 2012). In contrast, Western medical practices focus on targeting biochemical and genetic basis of the disease while alternative medical practices are typically left to the periphery of patient care. Collaboration between the Western and Eastern models of disease and treatment hinges on underst and ing which key elements found in TCM are essential to producing the beneficial therapeutic effects. Creating knowledge databases that dissect the interrelationships of individual ingredients could lead to novel approaches to both prevention and treatment of human diseases(McNamara, 2012; Jiang, 2005; Li CG, 2003; Chan et al, 2010). The complexity of herbal ingredients commonly found in CHM treatments often includes both toxic as well as multiple interacting efficacious compounds that complicate the breakdown of pharmaceutical entities in the search for individual drugs. A few examples of successful drug development from CHM include antimalarial drug artemisinin that was extracted from Artemisia annua L. by cold water(Chen et al, 2012; Tu et al, 1981; Klayman, 1985; Tu, 2011; You-You et al, 1982), and FDA approved arsenic trioxide(trisenox)that was traditionally used to treat acute promyelocytic leukemia(APL)(Kwong and Todd, 1997; Lengfelder et al, 2012; Leu and Mohassel, 2009; Tamm et al, 1999; Mathews et al, 2011).
TCM, in particular herbal treatments, has been gaining popularity across the world(Hesketh and Zhu, 1997; Chen et al, 2012). The Chinese market for herbal products totals around 14 billion US dollars last measured in 2005, and is continuing to grow(Chen et al, 2012). In the United States and Europe, the acupuncture and herbal treatments are becoming commonplace, along with meditation and other traditional Eastern methods of disease treatment. In response to the popularity and awareness of alternative medicine, the United States National Institutes of Health(NIH)established the National Center for Complementary and Alternative Medicine(NCCAM)to fund basic and clinical research on alternative therapies(Chan et al, 2010; NIH, 2012), which supported the initial research on Sargassum fusiforme(Harv.)Setchel described in this review.
The holistic aspect of TCM has led to many claims regarding its effectiveness at treating complex multisystem diseases based on the concept that human disease does not simply reflect perturbation of body(organ)system. In China, many healthcare providers employ TCM alone or in conjunction with Western medicine when providing care for patients, requiring education of physicians in both TCM as well as Western medical therapeutic principles(Hesketh and Zhu, 1997; McNamara, 2012; Jiang, 2005). Based on our results with S. fusiforme summarized in this review, a clinical trial is being performed at the First Affiliated Hospital of Henan University of Traditional Chinese Medicine in Zhengzhou, China. In this clinical trial, patients with AIDS are r and omized to treatment with st and ard highly active antiretroviral therapy(HAART)regimens or HAART supplemented with a formulation of S. fusiforme(Dr. Hui-jun Guo, personal communication). Results from this trial are pending at the time of this review.
Rational drug discovery and development of compounds from CMM and CHM start with bioactivity-guided isolation of compounds followed by rigorous scientific testing, including target identification, toxicology, and preclinical testing of the efficacy in appropriate animal models of human disease. Bioactive molecules in which the target and mechanism of action are identified then become promising lead c and idates for additional preclinical and eventually human trials(Fischbach and Walsh, 2009; Milligan et al, 2013; Borris, 1996). Our approach was to underst and the detailed structure-activity relationship(SAR)using in silico modeling, de novo design and synthesis, and in vitro testing to design compounds with the desired therapeutic index(TI) and pharmacokinetic profile(Fidock et al, 2004; Jorgensen, 2009;Mandal et al, 2009). Combining such mechanistic approaches together with TCM practices will improve the underst and ing of CMM effects and as demonstrated with S. fusiforme, and can lead to human testing of novel agents to prevent HIV infection in humans.
In this review, utilizing the paradigm of bridging Eastern and Western medicine of drug discovery and development from CHM, we describe the discovery of S. fusiforme as a potent inhibitor of HIV infection and detail the bioactive components, mechanism of action, and ongoing drug development strategy. 2. CD4 Inhibition and drug development for HIV infection
Our efforts target is CD4, the primary cellular receptor for HIV entry. The expression of CD4 on the target cells is necessary but not sufficient for HIV entry and infection. Several chemokine receptors [CXCR4(X4) and CCR5(R5)] act as co-factors that allow HIV entry when co-expressed with CD4 on the cell surface(Alkhatib et al, 1996; Feng et al, 1996; Deng et al, 1996; Dragic et al, 1996). The HIV tropism is defined in terms of the use of co-receptors during virus entry intothe target cells. Thus, HIV strains are defined as either R5-tropic, X4-tropic, or R5/X4 dual-tropic. Viral strains are predominantly R5-tropic, while during later stages of AIDS X4-tropic strains emerge further complicating patient treatment. However, the mechanism of viral entry seems to be similar regardless of tropism, and requires CD4 receptor binding regardless of co-receptor usage(Doms, 2001; Hori, 2001). HIV envelope protein(gp160)is composed of a highly variable extracellular portion(gp120) and a hydrophobic transmembrane part(gp41). HIV uses gp120 glycoprotein to bind to CD4 receptor(antigen)on cells such as T helper lymphocytes or macrophages. The gp120 and CD4 receptor interaction induces a conformational change in the gp120 molecule that is stabilized by attachment to either R5 or X4 co-receptor. Further conformational change exposes gp41 hydrophobic regions that enable the virion to embed(or fuse)into the host cell membrane. Once membrane fusion occurs with the host cell, the virion nucleocapsid(containing the RNA genome)is able to enter the cytoplasm of the cell and begin genomic RNA reverse transcription to cDNA, essentially initiating the formation of new virions.
Currently, there are two FDA-approved HIV inhibitors that block virus entry into the cell. Enfuvirtide blocks HIV fusion with the membrane at the gp41 stage while maraviroc binds to and blocks the R5 co-receptor(Dorr et al, 2005; Troia-Cancio and Asmuth, 2011; Kilby et al, 1998; Matthews et al, 2004). Maraviroc works only against R5 tropic viruses, and enfuvirtide is a large peptide that must be given by injection(Lu et al, 2006). These considerations and the clinical side effects limit the widespread use of these two entry inhibitors. Several previous investigations on targeting CD4 have had limited success due to the toxicity and disruption of CD4 function, which is a critical element of the host immune responses. TNX-355(Ibalizumab)is a non-immunosuppressive monoclonal antibody that binds to CD4 and inhibits the binding of gp120. Results from a Phase 2 clinical study show that the HIV infected patients receiving an optimized drug regimen have greater reduction in viral load and increased CD4 levels when treated in addition with TNX-355. BMS-488043, a small molecule that interferes with the interaction of CD4 and gp120 in Phase 1 clinical trials showed a modest reduction in viral loads(Hanna et al, 2011). Epigallocatechin gallate, a substance found in green tea, appears to interact with gp120 as do several other theaflavins(anti-oxidant polyphenols), and blocks virus entry byun known mechanisms(Williamson et al, 2006). The monoclonal antibody b12 has activity against HIV entry as originally found in long-term non-progressors. The activity against HIV entry has been shown to be related to disruption of binding of gp120 to a region on the surface of CD4, thereby serving as an entry inhibitor along the same lines of the compounds tested. Clinical trials of recombinant b12 antibody focused on prevention of HIV infection. Griffithsin, a substance derived from algae, appears to have entry inhibitor properties(Emau et al, 2007; O'Keefe et al, 2009). Recently, a unique approach using CD4 specific designed ankyrin repeat proteins(DARP)designed as alternatives to antibodies have been constructed to specifically and potently block with high affinity CD4 mediated viral entry across diverse strains of HIV. DARP in compounds are being developed clinically as potential microbicide c and idates that would prevent HIV infection following sexual activity(Schweizer et al, 2008).
The direct use of CD4 as a target for HIV inhibition addresses the limitation posed by prior efforts at blocking viral entry. Considering that the CD4 antigen is the obligatory receptor for HIV entry, it represents a desirable drug development target as it blocks de novo infection and reinfection in an already infected host. In blocking CD4, the particular viral tropism would not impact effectiveness, allowing for inhibition of a broad range of viral strains using a single drug. In addition, there is a lower theshold for viral evasion, because unlike the viral gp120, CD4 is a highly conserved human receptor therefore less likely to mutate as opposed to targeting viral envelope proteins, which are highly variable and have a high rate of mutation. However, because of its fundamental immunomodulatory role, it is essential thatthedrug development targeting CD4 receptor does not affect its primary immunomodulatory function.
Our strategy involves the development of small chemical entities originally isolated from S. fusiforme that specifically targets CD4 receptor and inhibits otherwise efficient virus gp120-to-CD4 binding, and that does not perturb the innate immune function of CD4 positive cells. 3. Inhibition of aqueous extract from S. fusiforme on HIV infection
At the 11th International Conference on AIDS in 2013, Dr. Peter Piot, UNAIDS Executive Director, called for the study and use of traditional medicines as a potential source of treatment, especially in developing countries. Products derived from natural sources have been shown to inhibit HIV-1 replication during various stages of the virus life cycle, and therefore represent a potential source of novel therapeutic agents(Chang and Yeung, 1988; Collins et al, 1997; Li and Vederas, 2009; Min et al, 1999; Oh et al, 2011; Park et al, 2009; Schaeffer and Krylov, 2000). To exp and our arsenal of therapeutics against HIV-1 infection and to identify potential lead compounds, we tested whole aqueous extracts prepared from 10 different natural products from TCM repertoire against HIV infection such asBupleurum falcatum, Crataegus pinnitifida, Forsythia suspensa, Glechoma longituba, Isatis tinctoria, Magnolia officnalis, Polyporus umbellatus, Prunella vulgaris, Rheum officinale baill, and Sargassum fusiforme. Different TCM products were chosen based on their antimicrobial or illness-fighting properties, therefore indicating possible antiviral activity(Hou, 2005; 1999). We found that only C. pinnitifida, F. suspensa, and S. fusiforme inhibited HIV infection by > 50% on day 3, and by > 80% on days 5 and 7 after in vitro infection of T cells. Inhibition between 50% and 80% suggests that these natural products contain potent antiviral activity, however, based on their relatively low efficacy, they were not considered viable c and idates for further testing. Only S. fusiforme consistently inhibited HIV infection by over 90% that was similar to inhibition by control nucleoside analogue 2′, 3′-didoxycytidine(ddC). Time course of HIV-1 inhibition in 1G5 T cells treated with whole S. fusiforme(2 mg/mL)or with 10-6 mol/L ddC control, and infected for 3 h with HIV-1(NL4-3)molecular clone(Figure 1A). S. fusiformeis a species of brown macroalgae(Phaeophyceae)that is commonly found in middle to lower rocky intertidal zones along the coastlines of China, Korea, and Japan. Formerly called Hizikia fusiformis, it frequently occurs in dense aggregations. Individual branches can be up to 1 m in length, with shorter side branches and narrow blades(Dong et al, 2002; Yokoi and Konomi, 2012). It is frequently collected for human consumption, raw or cooked. S. fusiforme has been studied for the ability to treat leukemia and herpes, indicating that it may contain multiple bioactive molecules(Chen et al, 2012; Chan et al, 2010; Lee et al, 2004).
Considering the observed high level and consistent HIV inhibition by whole S. fusiformeextract, we decided to conduct further studies, which demonstrated dose dependent inhibition of cell-to-cell mediated viral spread as well as inhibition of virus-to-cell infection. We demonstrated inhibition in T cell lines, and in primary human PBMCs, macrophages, microglia, and astrocytes that do not express CD4 receptor(Paskaleva et al, 2006). S. fusiforme only partially inhibited HIV infection in human CD4-negative astrocytes, or in T cells infected with HIV pseudotyped with VSV-G envelope that bypasses CD4 receptor restriction, suggesting that the primary mode of action was at the point of entry of HIV. However, the data also suggested that there were additional moieties that might be affecting other elements of the virus life cycle. Based on these results we selected S. fusiforme for further study to identify its bioactive components and to determine whether the specific mechanism of inhibition was at the viral entry into the cell. Utilizing bioactivity-guided fractionation, we produced over 600 fractions that were tested for HIV inhibition. One fraction(SP4-2)inhibited HIV infection with 230-fold efficacy enhancement as compared to the whole extract of S. fusiforme(Paskaleva et al, 2008). Results with SP4-2 fraction showed inhibition that was reversed by addition of competitor soluble CD4(sCD4)molecule, suggesting direct block of CD4 receptor during virus entry by up to 53%, and this also explained the observed inhibition of both R5- and X4-tropic HIV strains. SP4-2 inhibited HIV replication after entry as well as by directly inhibiting HIV reverse transcriptase(RT)in a dose dependent manner by up to 79%. These results suggested that SP4-2 fraction contained at least two bioactive molecules that inhibited HIV infection during entry and post-entry events of virus life cycle, and that confirmed S. fusiforme as a lead c and idate for further drug development. 4. Isolation of bioactive molecules from S. fusiforme and their mechanism of action
From the SP4-2 fraction, we isolated and identified two unsaturated fatty acids, oleic acid and linoleic acid, and twosaturated fatty acids, myristic acid, and palmitic acid(PA). Oleic acid and linoleic acid displayed anti-HIV properties by inhibiting RT in a dose dependent manner(Figures 1C and 1D), explaining the observed post-entry inhibition of infection by S. fusiforme. St and ard cell-free fluorescent RT assay was performed in the presence of two units of recombinant HIV-1 RT/reaction with the indicated concentration of oleic acid(C)
or linoleic acid(D). Myristic acid was ineffective at directly blocking HIV replication, although previous studies showed that myristoylation of the viral gag protein is necessary during virus assembly and egress(Lindwasser and Resh, 2002). Based on our previous results with S. fusiforme and SP4-2 fraction, which indicated HIV inhibition of entry and post-entry events, we decided to focus on palmitic acid as a potential inhibitor of HIV entry. Human PBMCs were treated overnight with increasing concentration of PA or with 10-6 mol/L ddC positive control for inhibition, washed, and infected with NL4-3 at 0.1 MOI(multiplicity of infection)for 2 h in the absence of each treatment, washed for three times, and returned to culture with each respective treatment for the duration of the experiment(Figure 1B).
PA, a molecule that has not been previously tested for its antiviral properties, inhibited the direct virus-to-cell entry and infection, and it also inhibited gp120-to-CD4 binding in a dose dependent manner(Lee et al, 2009). Inhibition of infection by both X4- and R5-tropic HIV strains was observed in primary PBMCs and macrophages, which were the main HIV target cells in vivo. We used one-dimensional saturation transfer difference NMR(STD-NMR)to show that PA binds directly to the CD4 receptor(Figure 2), thus establishing a mechanism of action at the point of HIV entry(Lee et al, 2009). The STD-NMR results showed that PA binding epitope for CD4 consisted of hydrophobic methyl and methylene groups located away from PA carboxyl terminal, which blocked efficient gp120-CD4 attachment. Collectively these results demonstrated that PA binds directly to the CD4 receptor and inhibited HIV entry and subsequent infection by blocking gp120-to-CD4 binding.
In Figure 2A, the homonuclear NMR spectrum of free 100 µmol/L PA was obtained in NMR buffer(consists of 10 mmol/L KPO4 buffer, pH 7.0, 20% DMSO-d6, 80% D2O). Thirteen PA methylene groups located away from the carboxyl terminal resonate at δ 1.5. PAmethylene groups located close to the PA carboxyl terminal resonate at δ 2.27 and 1.57, respectively. As shown in Figure 2B, STD-NMR signal of PA bound to sCD4. The increase of methylene STD-NMR signal of PA at δ 1.5 is observed with the increase of PA concentration in the sample of 14 µmol/L sCD4 dissolved in NMR buffer:(1)no PA, (2)molar ratio of sCD4 to PA is 1: 0.1, (3)1:0.6, (4)1: 0.8, (5)1:1.2, (6)1: 2, (7)1:3, (8)1:5, (9)1:7, and (10)1:10. The non-zero STD-NMR signal of PA indicates that PA directly binds to sCD4. PA methylene groups -(CH2)13- that show large STD-NMR signal constitute to the binding epitope of PA for CD4. Peaks from the PA methylene groups located close to the PA carboxyl terminal that are not part of the binding epitope are suppressed in the STD-NMR spectrum. Figure 2C shows thefractional STD effect of the -(CH2)13- signal at a given PA concentration. Gradual decrease of the STD effect indicates that the PA-sCD4 complex is specific. Figure 2D shows the result of the fluorescence titration experiment of sCD4 with increasing concentration of PA. This experiment was used to estimate the binding affinity of PA for sCD4. Tryptophan fluorescence was measured using an excitation wavelength of 280 nm. An increase of PA causes a red shift of 2 nm and quenching of the tryptophan fluorescence of sCD4. Insert: Binding isotherm of the normalized sCD4 tryptophan fluorescence with increasing concentration of PA at the emission wavelength of 350 nm. Curve fitting(OriginLab)using a single site binding isotherm approximation resulted in the best value for Kd to be(1.5 ± 0.2)µmol/L.
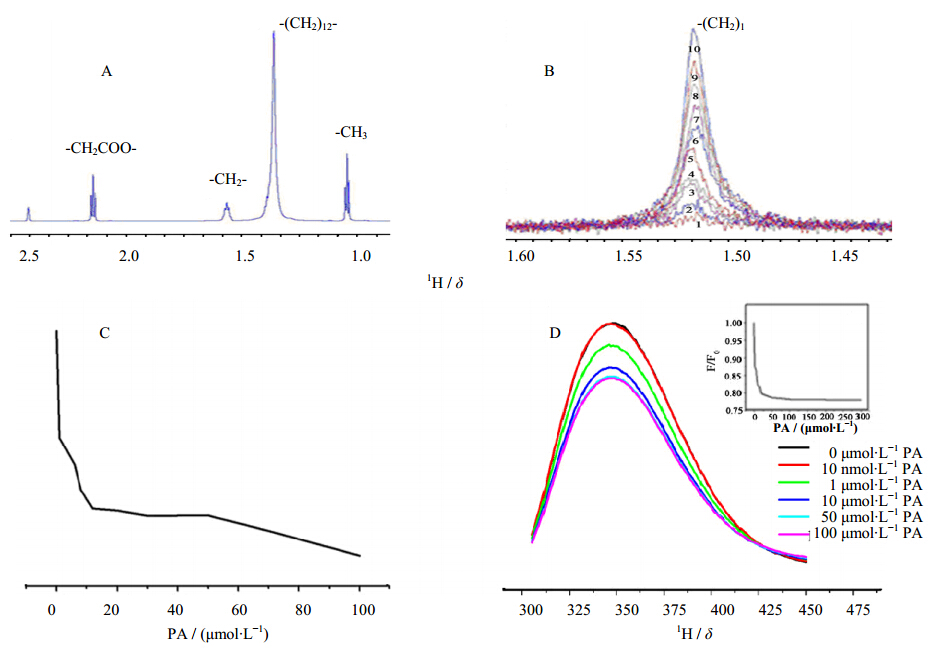 | Figure 2 In-vitro binding experiments and analysis of sCD4 with PA |
Next we wanted to measure the clinical efficacy of PA to inhibit HIV infection particularly during sexual transmission, which accounted for approximately 80% of all new infections with women bearing a disproportionate burden of people infected with HIV following sexual exposure(Klasse et al, 2008). In the absence of an effective vaccine, there is an urgent need to supplement currently available strategies with novel therapeutics including microbicides that are aimed at preventing sexual transmission of HIV. Viral entry inhibitors are ideally suited for use in microbicide formulations(Hoffman et al, 2008; Klasse et al, 2008; Ramjee et al, 2008). However currently there are no clinically approved microbicides or clinical trials that specifically include CD4 inhibitors of virus entry(for a full list at www.avac.org). We tested the ability of PA to inhibit HIV-1 infection in cervical tissue ex vivo model of human vagina, and in the underlying submucosa primary target cells, peripheral blood lymphocytes(PBL) and macrophages(Mø’s). Treatment with PA inhibited active X4 and R5 virus replication at the peak of infection and in both cell types up to 82% and 98%, respectively. Collectively, these results dem onstrated the inhibition of productive infection in human cervix tissue ex vivo model experiments, demonstrating opportunity for topical microbicide development aimed at preventing HIV sexual transmission(Lin et al, 2011), which remains the main cause of new HIV infections. 5. TCM, HIV entry inhibition, and drug development
The sub-micromolar inhibition level limits the clinical effectiveness of PA. To this end, we were interested in investigating whether PA could be used as a structural model molecule for unlocking novel gp120 binding sites on the CD4 molecule, thereby enabling chemical modifications of the PA scaffold model in order to improve its potency to nanomolar range. We reasoned that underst and ing the structure-affinity relationship(SAR)between PA and CD4 could lead to development of PA analogs with greater potency in blocking HIV-1 entry. Considering PA’s molecular structure and bifunctional mechanism of inhibition, we modeled in silico this structure-activity relationship, and searched chemical databases for PA analogs that would satisfy Lipinski’s rules of drug-likeness(Lipinski et al, 2001; Paskaleva et al, 2010). We constructedin silico model of PA-CD4 binding and gp120 interaction and we identified a CD4 cavity that was formed by amino acids Phe52, Leu60, Leu62, Leu63, and Leu70, that had a tight fit for palmitic acid(Figure 3). As shown in Figure 3A, molecular docking software Autodock 4.0 was used for blind docking of flexible PA onto rigid two N-terminal domains of CD4(PDB code 1GC1). The resultant PA-CD4 conformations
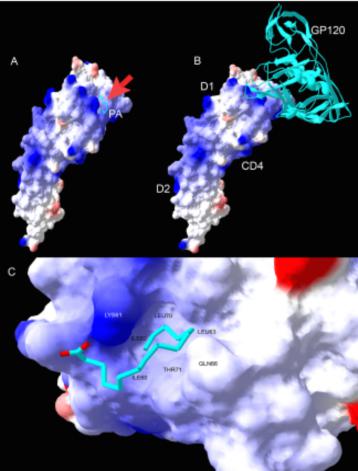 | Figure 3 PA-CD4-gp120 interaction model |
were ranked and categorized based on the value of free energy of binding. Docking runs 386 out of 1000fell into conformations that are ranked with the highest score. The root mean square deviation of these conformations was 1.2. Figure 3A suggests very similar binding modes.
One of the lig and bound conformations of PA-CD4 with a highest score is shown in cyan(PA aliphatic chain) and red(PA carboxylic terminus). Close-up of the PA-CD4 binding cavity is shown in Figure 3A. The crystal structure of gp120-CD4(PDB code 1GC1)is shown in Figure 3B. Comparison between PA-CD4 and gp120-CD4 structures shows the overlapping binding sites for gp120 and PA. In Figure 3C, PA occupies a hydrophobic cavity formed by Phe52, His53, Lys61, and Leu 63 of CD4. Electrostatic potential calculated using DelPhi software was mapped onto the molecular surface of CD4. Positively and negatively charged surfaces are in blue and red, respectively. Non-polar surface is in white. PA was depicted using wireframe model with aliphatic chain in cyan and carboxyl terminal in red. We used Discover Studio software(AccelRys)to prepare this figure. Of the PA analogs tested for inhibition of HIV entry, 2-bromopalmitate(2-BP)was most efficacious in CD4 binding and it inhibited CD4-gp120 binding with nanomolar efficacy. In addition, while PA forms micelles at higher micromolar concentration, we demonstrated that 2-BP bound to CD4 with a 1:1 binding stoichiometry. This result signifies that 2-BP inhibits HIV-1 fusion by binding to the CD4 receptor as a single molecule, and not in the form of micelles that exist at higher micromolar concentration. Taken together, these results demonstrated that the identified CD4 receptor cavity binding PA and 2-BP is a druggable cavity, and PA and 2-BP represent useful scaffold model molecules in defining structure-activity relationship. We are utilizing this platform and further identifying more potent small chemical entities for development and optimization that would mitigate HIV transmission at the point of contact.
Once a lead molecule is identified and the structure- activity relationship is understood, in silico modeling, de novo design and synthesis, and in vitro testing can be rapidly performed to achieve the desired therapeutic index(TI) and pharmacokinetic profile. This type of structural model-based approach is used extensively in basic research to identify more potent compounds(Hajduk and Greer, 2007; Fischbach and Walsh, 2009). To validate the proposition for drug development, we tested the PA and 2-BP scaffold molecules for genotoxic potential(Paskaleva, 2014). The FDA and the International Conference on Harmonization(ICH)recommends using a st and ardized 3-test battery for testing compound genotoxicity consisting of the bacterial reverse mutation assay, mouse lymphoma assay, and rat micronucleus assay. PA, 2-BP, and their metabolites tested negative for toxicity in all three tests. Collectively, based on the results we concluded that PA and 2-BP model scaffolds are safe for further development of HIV entry inhibitors with similar structures. 2-BP is the first derivative of PA to undergo preclinical screening, which will enable us to develop and test multiple small chemical structures based on their anti-HIV activity in scaffold modeling across the dimension of pre-clinical testing to enable transition to human testing. 6. Conclusion
Ideally, bridging the two paradigms between Eastern and Western medicine involves testing of TCM compounds against a specific disease, identification of bioactive molecules and their mechanism of action, modeling the disease in vitro and using animal models, preclinical testing, and finally lead c and idate testing in human trials. Applying this approach to TCM therapy has been very successful to date, and holds the promise to mine a rich source of natural compounds with long-st and ing medicinal clinical history into pharmaceutical molecules that could target specific pathways across an array of human diseases. In our case example, described in this review, our screening identified a potent inhibitor of HIV replication from S. fusiforme, and we were able to differentiate several novel inhibitors of HIV infection within this TCM natural product. We identified the mechanism of action, and are currently exploring whether structure-activity modeling can be used to create more potent inhibitors to the CD4 cleft. Collectively, these studies demonstrate a synergistic approach between two different models: clinical testing of S. fusiforme as part of TCM therapeutic against HIV/AIDS in China, and further drug development by optimization of CD4-based HIV binding inhibitors that do not adversely affect CD4 function.
| [1] | Alkhatib G,Combadiere C,Broder CC,Feng Y,Kennedy PE,Murphy PM,Berger EA. 1996. CC CKR5: a RANTES,MIP-1alpha,MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955-1958. |
| [2] | Borris RP,1996. Natural products research: perspectives from a major pharmaceutical company.J Ethnopharmacol 51: 29-38 . |
| [3] | Chan E,Tan M,Xin J,Sudarsanam S,Johnson DE,2010. Interactions between traditional Chinese medicines and Western therapeutics. Curr Opin Drug Discov Deve 13:50-65 . |
| [4] | Chang RS,Yeung HW,1988. Inhibition of growth of human immunodeficiency virus in vitro by crude extracts of Chinese medicinal herbs. Antiviral Res 9: 163-175 . |
| [5] | Chen X,Nie W,Yu G,Li Y,Hu Y,Lu J,Jin L,2012. Antitumor and immunomodulatory activity of polysaccharides from Sargassum fusiforme. Food Chem Toxicol 50:695-700 . |
| [6] | Collins RA,Ng TB,Fong WP,Wan CC,Yeung HW,1997. A comparison of human immunodeficiency virus type 1 inhibition by partially purified aqueous extracts of Chinese medicinal herbs. Life Sci 60: PL345-351 . |
| [7] | Deng H,Liu R,Ellmeier W,Choe S,Unutmaz D,Burkhart M,Di Marzio P,Marmon S,Sutton RE,Hill CM,Davis CB,Peiper SC,Schall TJ,Littman DR,Landau NR,1996. Identification of a major co-receptor for primary isolates of HIV-1. Nature 381: 661-666 . |
| [8] | Doms,RW,2001. Chemokine receptors and HIV entry. AIDS 15 Suppl 1:S34-35 . |
| [9] | Dong Y,Li Y,Cui Z,Zhang Z,Liu D,Wang C,2002. Description and histology identification of several algae of Sargassum sp. J Chin Med Mater 25:239-242 . |
| [10] | Dorr P,Westby M,Dobbs S,Griffin P,Irvine B,Macartney M,Mori J,Rickett G,Smith-Burchnell C,Napier C,Webster R,Armour D,Price D,Stammen B,Wood A,Perros M,2005. Maraviroc (UK-427,857),a potent,orally bioavailable,and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob Agents Chemother 49: 4721-4732 . |
| [11] | Dragic T,Litwin V,Allaway GP,Martin SR,Huang Y,Nagashima KA,Cayanan C,Maddon PJ,Koup RA,Moore JP,Paxton WA,1996. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381:667-673 . |
| [12] | Emau P,Tian B,O'keefe BR,Mori T,Mcmahon JB,Palmer KE,Jiang Y,Bekele G,Tsai CC,2007. Griffithsin,a potent HIV entry inhibitor,is an excellent candidate for anti-HIV microbicide. J Med Primatol 36:244-253 . |
| [13] | Feng Y,Broder CC,Kennedy PE,Berger EA,1996. HIV-1 entry cofactor: Functional cDNA cloning of a seven-transmembrane,G protein-coupled receptor. Science 272:872-877 . |
| [14] | Fidock DA,Rosenthal PJ,Croft SL,Brun R,Nwaka S,2004. Antimalarial drug discovery: Efficacy models for compound screening. Nat Rev Drug Discov 3:509-520 . |
| [15] | Fischbach MA,Walsh CT,2009. Antibiotics for emerging pathogens. Science 325:1089-1093 . |
| [16] | Hajduk PJ,Greer J. 2007. A decade of fragment-based drug design: Strategic advances and lessons learned. Nat Rev Drug Discov 6: 211-219. |
| [17] | Hanna GJ,Lalezari J,Hellinger JA,Wohl DA,Nettles R,Persson A,Krystal M,Lin P,Colonno R,Grasela DM,2011. Antiviral activity,pharmacokinetics,and safety of BMS-488043,A novel oral small-molecule HIV-1 attachment inhibitor,in HIV-1-infected subjects. Antimicrob Agents Chemother 55: 722-728 . |
| [18] | Hesketh T,Zhu WX,1997. Health in China. Traditional Chinese medicine: One country,two systems. BMJ 315: 115-117. |
| [19] | Hoffman S,Cooper D,Ramjee G,Higgins JA,Mantell JE,2008. Microbicide acceptability: Insights for future directions from providers and policy makers. AIDS Educ Prev 20:188-202 . |
| [20] | Hori T,2001. [HIV-1 cell entry mediated by chemokine receptors]. Rinsho Ketsueki 42:267-272. |
| [21] | Hou JP,Jin YY,2005. The Healing Power of Chinese Herbs and Medicinal Recipes.Haworth Integrative Healing, New York . |
| [22] | Jiang WY,2005. Therapeutic wisdom in traditional Chinese medicine: A perspective from modern science. Trends Pharmacol Sci 26:558-563 . |
| [23] | Jorgensen WL,2009. Efficient drug lead discovery and optimization. Acc Chem Res 42:724-733 . |
| [24] | Kaptchuk T,2000. The Web That Has No Weaver: Understanding Chinese Medicine. NY,McGraw-Hill,New York . |
| [25] | Kilby JM,Hopkins S,Venetta TM,Dimassimo B,Cloud GA,Lee JY,Alldredge L,Hunter E,Lambert D,Bolognesi D,Matthews T,Johnson MR,Nowak MA,Shaw GM,Saag MS,1998. Potent suppression of HIV-1 replication in humans by T-20,a peptide inhibitor of gp41-mediated virus entry. Nat Med 4:1302-1307 . |
| [26] | Klasse PJ,Shattock R,Moore JP,2008. Antiretroviral drug-based microbicides to prevent HIV-1 sexual transmission. Annu Rev Med 59:455-471 . |
| [27] | Klayman DL,1985. Qinghaosu (artemisinin): An antimalarial drug from China. Science 228:1049-1055 . |
| [28] | Kwong YL,Todd D,1997. Delicious poison: Arsenic trioxide for the treatment of leukemia. Blood 89:3487-3488 . |
| [29] | Lee DY,Lin X,Paskaleva EE,Liu Y,Puttamadappa SS,Thornber C,Drake JR,Habulin M,Shekhtman A,Canki M,2009. Palmitic acid is a Novel CD4 fusion inhibitor that blocks HIV entry and infection. AIDS Res Hum Retroviruses 25:1231-1241 . |
| [30] | Lee JB,Hayashi K,Maeda M,Hayashi T,2004. Antiherpetic activities of sulfated polysaccharides from green algae. Planta Med 70:813-817 . |
| [31] | Lengfelder E,Hofmann WK,Nowak D,2012. Impact of arsenic trioxide in the treatment of acute promyelocytic leukemia. Leukemia 26:433-442 . |
| [32] | Leu L,Mohassel L,2009. Arsenic trioxide as first-line treatment for acute promyelocytic leukemia. Am J Health Syst Pharm 66:1913-1918 . |
| [33] | Li CG,Moyle K,Xue CC,2003. Problems and Challenges of Chinese Herbal Medicine. World Scientific Publishing Co., River Edge. |
| [34] | Li JW,Vederas JC,2009. Drug discovery and natural products: End of an era or an endless frontier? Science 325: 161-165 . |
| [35] | Lin X,Paskaleva EE,Chang W,Shekhtman A,Canki M,2011. Inhibition of HIV-1 infection in ex vivo cervical tissue model of human vagina by palmitic acid; implications for a microbicide development. PLoS One 6:e24803 . |
| [36] | Lindwasser OW,Resh MD,2002. Myristoylation as a target for inhibiting HIV assembly: Unsaturated fatty acids block viral budding. Proc Natl Acad Sci USA 99:13037-13042 . |
| [37] | Lipinski CA,Lombardo F,Dominy BW,Feeney PJ,2001. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 46: 3-26 . |
| [38] | Lu J,Deeks SG,Hoh R,Beatty G,Kuritzkes BA,Martin JN,Kuritzkes DR,2006. Rapid emergence of enfuvirtide resistance in HIV-1-infected patients: Results of a clonal analysis. J Acquir Immune Defic Syndr 43:60-64 . |
| [39] | Mandal S,Moudgil M,Mandal,SK,2009. Rational drug design. Eur J Pharmacol 625:90-100 . |
| [40] | Mathews V,Chendamarai E,George B,Viswabandya A,Srivastava,A,2011. Treatment of acute promyelocytic leukemia with single-agent arsenic trioxide. Mediterr J Hematol Infect Dis 3: e2011056 . |
| [41] | Matthews T,Salgo M,Greenberg M,Chung J,Demasi R,Bolognesi D,2004. Enfuvirtide: The first therapy to inhibit the entry of HIV-1 into host CD4 lymphocytes. Nat Rev Drug Discov 3: 215-225 . |
| [42] | Mcnamara S,Ke SX,2012. Traditional Chinese Medicine. In: PALMER,A. (ed.). |
| [43] | Men JZ,Guo L,2009. A General Introduction to Traditional Chinese Medicine. CRC Press,China . |
| [44] | Milligan PA,Brown MJ,Marchant B,Martin SW,Van Der Graaf PH,Benson N,Nucci G,Nichols DJ,Boyd RA,Mandema JW,Krishnaswami S,Zwillich S,Gruben D,Anziano RJ,Stock TC,Lalonde RL,2013. Model-based drug development: A rational approach to efficiently accelerate drug development. Clin Pharmacol Ther 93:502-514 . |
| [45] | Min BS,Jung HJ,Lee JS,Kim YH,Bok SH,Ma CM,Nakamura N,Hattori M,Bae K,1999. Inhibitory effect of triterpenes from Crataegus pinatifida on HIV-I protease. Planta Med 65:374-375 . |
| [46] | NIH. 2012. Traditional Chinese Medicine [Online]. National Center for Complementary and Alternative Medicine. Available: http://nccam.nih.gov/health/whatiscam/chinesemed.htm [Accessed 15 October 2013]. |
| [47] | O'brien Ka XC,2003. The Theoretical Framework of Chinese Medicine. World Scientific Publishing Co., River Edge. |
| [48] | O'keefe BR,Vojdani F,Buffa V,Shattock RJ,Montefiori DC,Bakke J,Mirsalis J,D'andrea AL,Hume SD,Bratcher B,Saucedo CJ,Mcmahon JB,Pogue GP,Palmer KE,2009. Scaleable manufacture of HIV-1 entry inhibitor griffithsin and validation of its safety and efficacy as a topical microbicide component. Proc Natl Acad Sci USA 106:6099-6104 . |
| [49] | Oh C,Price J,Brindley MA,Widrlechner MP,Qu L,Mccoy JA,Murphy P,Hauck C,Maury W,2011. Inhibition of HIV-1 infection by aqueous extracts of Prunella vulgaris L. Virol J 8: 188 . |
| [50] | Park IW,Han C,Song X,Green LA,Wang T,Liu Y,Cen C,Song X,Yang B,Chen G,He JJ,2009. Inhibition of HIV-1 entry by extracts derived from traditional Chinese medicinal herbal plants. BMC Complement Altern Med 9: 29 . |
| [51] | Paskaleva E,Arra M,Lui Y,Guo H,Swartz G,Breneman C,Shekhtman A,Canki M,2014. Evaluation of potential genotoxicity of HIV entry inhibitors derived from natural sources. PLoS One 9(3): e93108 . |
| [52] | Paskaleva EE,Lin X,Duus K,Mcsharry JJ,Veille JC,Thornber C,Liu Y,Lee DY,Canki M,2008. Sargassum fusiforme fraction is a potent and specific inhibitor of HIV-1 fusion and reverse transcriptase. Virol J 5: 8 . |
| [53] | Paskaleva EE,Lin X,Li W,Cotter R,Klein MT,Roberge E,Yu EK,Clark B,Veille JC,Liu Y,Lee DY,Canki M,2006. Inhibition of highly productive HIV-1 infection in T cells,primary human macrophages,microglia,and astrocytes by Sargassum fusiforme. AIDS Res Ther 3: 15 . |
| [54] | Paskaleva EE,Xue J,Lee DY,Shekhtman A,Canki M,2010. Palmitic acid analogs exhibit nanomolar binding affinity for the HIV-1 CD4 receptor and nanomolar inhibition of gp120-to-CD4 fusion. PLoS One 5: e12168 . |
| [55] | Ramjee G,Doncel GF,Mehendale S,Tolley EE,Dickson K,2008. Microbicides 2008 conference: from discovery to advocacy. AIDS Res Ther 5: 19 . |
| [56] | Schaeffer DJ,Krylov VS,2000. Anti-HIV activity of extracts and compounds from algae and cyanobacteria. Ecotoxicol Environ Saf 45:208-227 . |
| [57] | Schweizer A,Rusert P,Berlinger L,Ruprecht CR,Mann A,Corthesy S,Turville SG,Aravantinou M,Fischer M,Robbiani M,Amstutz P,Trkola A,2008. CD4-specific designed ankyrin repeat proteins are novel potent HIV entry inhibitors with unique characteristics. PLoS Pathog 4: e1000109 . |
| [58] | Tamm I,Paternostro G,Zapata JM,1999. Treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med 340: 1043; author reply 1044-1045 . |
| [59] | Troia-Cancio P,Asmuth DM,2011. Lessons from maraviroc clinical trials. Expert Rev Anti Infect Ther 9: 649-651 . |
| [60] | Tu Y,2011. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat Med 17:1217-1220 . |
| [61] | Tu YY,Ni MY,Zhong YR,Li LN,Cui SL,Zhang MQ,Wang XZ,Liang XT,1981. [Studies on the constituents of Artemisia annua L. (author's transl)]. Acta Pharm Sin 16:366-370 . |
| [62] | Tu YY,Ni MY,Zhong YR,Li LN,ui SL,Zhang MQ,Wang XZ,Liang XT,1982. Studies on the constituents of Artemisia annua Part II. Planta Med 44:143-145 . |
| [63] | United Nations,2012. UNAIDS report on the global AIDS epidemic . |
| [64] | Williamson MP,Mccormick TG,Nance CL,Shearer WT,2006. Epigallocatechin gallate,the main polyphenol in green tea,binds to the T-cell receptor,CD4: Potential for HIV-1 therapy. J Allergy Clin Immunol 118: 1369-1374 . |
| [65] | Wiseman N,Ellis A,1996. Fundamentals of Chinese Medicine,ParadigmPublications Taos. |
| [66] | WHO,1999. WHO Monographs on Selected Medicinal Plants.Volume 1. Organizacion Mnudial De La Salud. Available: http://apps.who.int/medicinedocs/en/d/Js2200e/ [Accessed March 13 2013] . |
| [67] | Yokoi K,Konomi A,2012. Toxicity of so-called edible hijiki seaweed (Sargassum fusiforme) containing inorganic arsenic. Regul Toxicol Pharmacol 63:291-297. |
 2014, Vol. 6
2014, Vol. 6


