1. Introduction
Diabetes is one of the most prevalent chronic diseases in the world. During 2010 to 2030,there is an increase of 69% in the number of adults with diabetes in developing countries and an increase of 20% in developed countries(Shaw et al,2010). Diabetes is ultimately due to chronically high level of blood glucose. The persistent hyperglycemic condition in diabetes plays a predisposing role in dysfunction and failure of various organs(American Diabetes Association,2011). Thus,the sustained control of hyperglycemia is considered to be important to the effective treatment of diabetes. For a long time,diabetics has been treated by insulin and synthetic drugs,which are available at present,but they produced serious side effects. Hypoglycemic episodes cause black-outs syndrome and even life-threatening(Lopez-Candales, 2001). Herbal medicines play an important role in this part to prevent side effects. Located in the brush-border surface membrane of intestinal cells,α-glucosidase plays a crucial role in dietary carbohydrates digestion and post-translational processing of glycoprotein(Carrascosa et al,2001). By inhibiting the function of α-glucosidase,many herbal components can delay the glucose absorption as inhibitors(Andrade-Cetto et al,2008).
Blumea balsamifera(L.)DC.(BB)is a medicinal plant used as carminative,vermifuge,diaphoretic, and expectorant medicine in Southeast Asia and China. The studies on the constituents from BB were carried out and a number of flavones,monoterpenes, and triterpenes have been isolated from this plant(Nessa et al,2004). In Luodian county,Guizhou province,the major producing area of BB,the local farmers take the volatile oil from BB by steam distillation(Jiang et al,2006) and discard the waste residues ad libitum. This causes both the waste of resources and the environmental pollution.
In the ongoing research,we found the residues of BB could ameliorate diabetes. To explore the potentiality,we carried out assays to screen the active fractions hampering the glucose absorption in vivo and in vitro. In this paper,we report the active fractions screening results. The compounds were all assayed to determine whether they could inhibit the activity of α-glucosidase, and their inhibiting manner. So the study could extend the application field of BB residues and proper utilization of waste resource, and reduce the environmental pollution as well.
2. Materials and methods 2.1 Reagents and materialsThe leaves of Blumea balsamifera(L.)DC. were collected in Luodian county,Guizhou province,China. The plant was authenticated by Prof. Jian-wei Chen,College of Pharmacy,Nanjing University of Chinese Medicine,China. A voucher specimen(ID: ANX2013-315)was deposited in the Herbarium Center,Nanjing University of Chinese Medicine,China. α-Glucosidases from Saccharomyces cerevisiae,p-nitrophenyl-α-D-glucopyranoside(PNPG), and acarbose were purchased from Sigma-Aldrich(USA). Petroleum ether,ethyl acetate,butyl alcohol,methanol, and ethanol(for extraction and column chromatography)were purchased from Guanghua Sci-tech Co.,Ltd.(China). The reagent-grade sodium dihydrogen phosphate,disodium hydrogen phosphate, and sodium hydroxide were purchased from Xilong Chemical Factory(Guangdong,China). Ultra-pure water was prepared using a Millipore Milli–Q Purification System by Eped Science Co.,Ltd.(Jiangsu,China).
2.2 Experimental animalsMale mice(4-week old)were purchased from Medical Comparison Center of Yangzhou University,China. All animals were housed individually in a light-dark(12 h /12 h)cycle and temperature-controlled room with pelleted food and water available ad libitum. All animal procedures were approved by the Ethical Committee of Nanjing University of Chinese Medicine, and strictly in accordance with the guide for the care and use of laboratory animals(US National Research Council,2011).
2.3 Fraction preparationThe waste water was gathered after taking the volatile oil from BB by steam distillation. Then the water extract fraction(BBW)was concentrated to dryness.
The dried residues of BB leaves were extracted for three times with 95% alcohol, and filtered. The residues were re-extracted with 50% alcohol once. The filtrates were evaporated together by a rotation evaporator; The total extract was obtained and heated to dryness. Then it was partitioned with petroleum ether,ethyl acetate,butyl alcohol, and methanol in turns. Afterwards,each part was concentrated to dryness in vacuo to yield four fractions: petroleum ether fraction(BBP),ethyl acetate fraction(BBE),butyl alcohol fraction(BBB), and methanol fraction(BBM)for biological assay.
2.4 Inhibitory assay of fractions against α-glucosidaseThe α-glucosidase enzyme reaction was performed using PNPG as substrate. The inhibitory activity of enzyme was measured spectrophotometrically through monitoring the nitrophenyl produced by the substrate hydrolysis(Shelly et al,2010). α-Glucosidase,acarbose,sodium carbonate, and the four fractions(samples)were all dissolved with 0.1% PBS to dissolution individually. α-Glucosidase(20 μL,0.2 unit/mL)was treated with 10 μL of sample solutions for 15 min at 37 °C,using the same volume of PBS as a negative control and 20 mg/mL acarbose as a positive control. The final volume of reaction solution was 0.16 mL added with 0.1% PBS. PNPG(20 μL,2.5 mmol/L)was added to initiate the enzyme reaction. After incubating the mixture at 37 °C for 15 min,80 μL of sodium carbonate solution was used to cease the reaction. And then OD values were detected at 405 nm by plate reader(BIO-RAD Science Co.,Ltd.,USA).
Enzymatic inhibitory activity/% =(1 − Ax/A0)× 100%
where A0 represents the OD value of PBS control and Ax represents those of samples being tested
According to the preliminary results,similar experiments were undertaken at different concentration using the same method.
2.5 Inhibitory manner analyses of fractionsα-Glucosidase was treated with or without samples for 15 min at 37 °C and then the substrate(1.25,1.67,2.50,3.75,or 8.33 mmol/L PNPG)was added to initiate the enzyme reaction. The main procedures were the same as described above. Velocity of the reaction was observed for double reciprocal plots analysis(Wang et al,2013). The concentration of α-glucosidase inhibitor was 7.69,1.54,7.69,30.77, and 1.54 mg/mL for BBP,BBE,BBB,BBM, and BBW,respectively.
2.6 Suppression of fractions on serum glucose levels in normal miceNormal mice were r and omly divided into 17 groups. The mice(n = 8)were ig administered with various doses of fractions for 8 d. The mice of vehicle control were given 0.5% CMC-Na. After the last administration,the serum glucose levels were monitored at 1 and 12 h after feeding by tail bleeding using a glucometer(Roche Diagnostics Gmbh,Germany)to compare the differences of postpr and ial serum glucose and fasting serum glucose levels among groups(The mice were fasted for 12 h after last feeding). Fasted animals were deprived of food but allowed free access to water(Kazuhiro et al,2010).
The doses of fractions were in according with the percentages in the crude drug. The exact doses were as follows: BBP 64,32, and 16 mg/kg; BBE 21.6,10.8, and 5.4 mg/kg; BBB 8.0,4.0, and 2.0 mg/kg; BBM 10.4,5.2, and 2.6 mg/kg; BBW 184.8,92.4, and 45.2 mg/kg.
3. Results 3.1 Inhibition of fractions against α-glucosidaseThe fractions could inhibit the α-glucosidase activity. The inhibitory activities of different fractions and acarbose against α-glucosidase were detected in the same concentration of 0.77 mg/mL. All the fractions inhibited the α-glucosidase activity. BBW inhibited the activity by 73.9% and BBE by 50.9%,whereas BBP,BBB, and BBM showed relatively lower inhibitory activities of 15.5%,11.0%, and 1.9%. The IC50 values of BBP,BBE,BBB,BBW, and acarbose were 4.63,0.75,4.96,0.18, and 0.74 mg/mL,respectively. The inhibitory rates of BBM at all the concentration were below 50%(Table 1).
| Table 1 IC50 values of all fractions and acarbose |
The results of inhibitory manner analyses are shown in Figure 1. There were four kinds of inhibitory manners according to the results. The double reciprocal plots of BBP were a parallel,indicating that it acted as an anticompetitive inhibitor. Anticompetitive inhibition indicated that the concentration of substrate(Sm)decreased,so did the maximum reaction velocity(Vm); But the Vm/Sm was invariant. Intersection of BBB seated on the horizontal axis,indicating that they acted as a noncompetitive reversible type inhibitor. Noncompetitive reversible type inhibition indicated that the change of substrate concentration had no effect on the rate of inhibition. So Sm was invariant and Vm decreased. Intersection of the double reciprocal plots of BBE seated in the second-quadrant,indicating that it acted as a mixed-type inhibitor. Mixed-type inhibition indicated there was a coexistence of competitive reversible and non-competitive reversible inhibition. While intersections of BBM and BBW seated on the longitudinal axis,indicating that they act as a competitive reversible type inhibitor. Competitive inhibition indicated that the inhibitory effect increased with the inhibitor concentration increasing,but increase the substrate concentration could make the inhibitory degree decreased. The Sm increased, and Vm was invariant(Kazuhiro et al,2010).
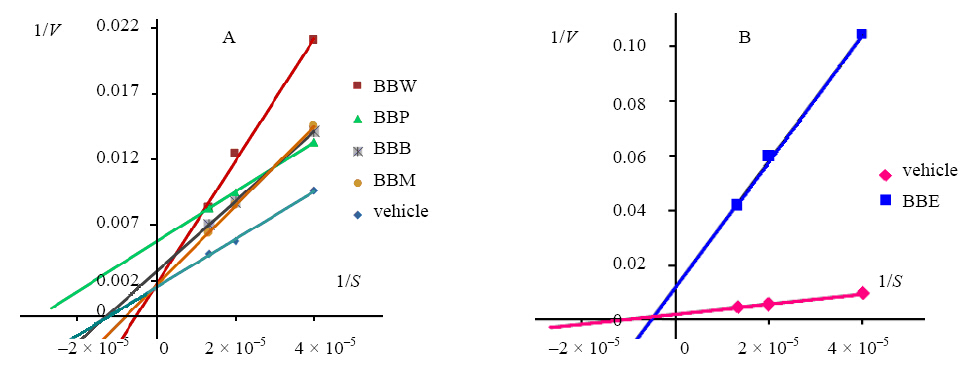
|
Figure 1 Inhibitory manners of BBP,BBB,BBM,BBW(A), and BBE(B) Horizontal axis: concentration of substrate Longitudinal axis: reciprocal of velocity |
All of the four fractions suppressed the serum glucose levels,both at fasting(Figure 2A) and postpr and ial conditions(Figure 2B). The result is similar with the assay of α-glucosidase inhibition.
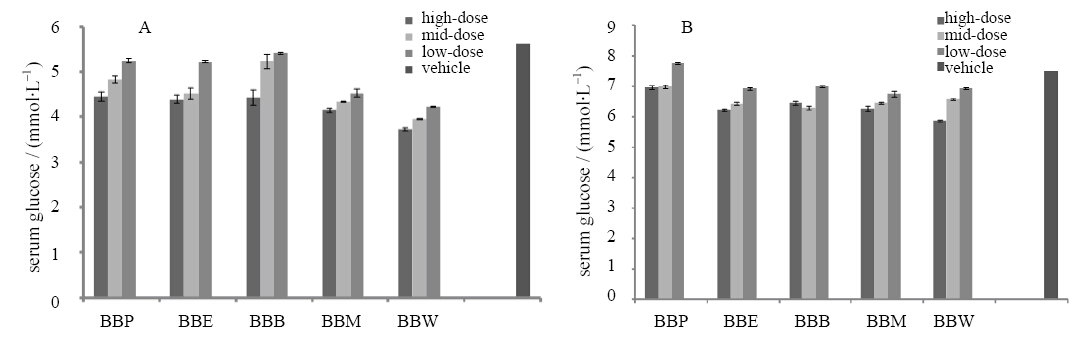
| Figure 2 Suppression of BBP,BBE,BBB,BBM, and BBW on serum glucose at fasting(A) and postpr and ial(B)conditions |
BBM had the best effect,while BBE and BBW were similar and suppressed the serum glucose levels efficiently. The other fractions exhibited weaker anti-hyperglycemic effects.
The inhibition assays against α-glucosidase exhibited that BBE and BBW had inhibitory activities similar to acarbose. BBE and BBW also showed a remarkable serum glucose levels suppressing activities compared with the control group,both at fasting and postpr and ial conditions in vivo. Based on these results,it was concluded that the residues of BB could ameliorate diabetes by slowing down the glucose absorption as α-glucosidase inhibitor. The present study was designed to extend the application field of BB residues,properly utilize the waste resource, and testify the potential anti-diabetic activity of BB by using enzyme assay in vitro and normal mice model in vivo.
5. ConclusionWe investigate the residues of BB as potential anti- diabetes medicine for the first time. All the fractions from BB residues have been found to suppress the serum glucose levels in the normal mice. In addition,BBE and BBW could efficiently inhibit the α-glucosidase activity. In the future we will attempt to find out the active compounds in it.
| [1] | American Diabetes Association,2011. Diagnosis and classification of diabetes mellitus.Diabetes Care 33 (Suppl 1): 62-69. |
| [2] | Andrade-Cetto A,Becerra-Jimenez J,Cardenas-V R,2008. Alfa- glucosidase-inhibiting activity of some Mexican plants used in the treatment of type 2 diabetes. J Ethnopharmacol 116: 27-32. |
| [3] | Carrascosa JM,Molero JC,Fermin Y,Martinez C,Andres A,Satrustegui J,2001. Effects of chronic treatment with acarbose on glucose and lipid metabolism in obese diabetic wistar rats. Diabetes Obes Metab 3: 240-248. |
| [4] | Hogan S,Zhang L,Li J,Sun S,Canning C,Zhou K,2010.Antioxidant rich grape pomace extract suppresses postprandial hyperglycemia in diabetic mice by specifically inhibiting alpha-glucosidase. Nutr Metab 7: 71. |
| [5] | Jiang XL,Pan JF,Si J,2006. The Blumea Balsamifera powder: Technical research of reaping and abstracting in plantation area. Biomass Chem Eng 10: 17. |
| [6] | Kazuhiro W,Keiko K,Juichi S,Tunehisa T,2010. Fundamental studies on the inhibitory action of Acanthopanax senticosus Harms on glucose absorption. J Ethnopharmacol 132: 193-199. |
| [7] | Lopez-Candales A,2001. Metabolic syndrome X: A comprehensive review of the pathophysiology and recommended therapy. J Med 32(5/6): 283-300. |
| [8] | Nessa F,Ismail Z,Mohamed N,Mas Haris MRH,2004. Free radical-scavenging activity of organic extracts and of pure flavonoids of Blumea balsamifera DC leaves. Food Chem 88: 243-252. |
| [9] | Shaw JE,Sicree RA,Zimmet PZ,2010. Global estimates of the prevalence of diabetes for 2010 and 2030. Diab Res Clin Pract 87: 4-14. |
| [10] | Wang ZW,Wang JS,Luo J,Kong LY,2013. α-Glucosidase inhibitory triterpenoids from the stem barks of Uncaria laevigata. Fitoterapia 90: 30-37. |
 2014,Vol. 6
2014,Vol. 6


