2. Xinjiang Institute of Materia Medica, Urumqi 830002, China
3. Institute of Materia Medica, Chinese Academy of Medical Science, Beijing 100050, China
1. Introduction
Cardiovascular disease(CVD)is a leading cause of death, and hypertension is one of the most common causes of cardiovascular and cerebrovascular complications in human(Tang et al,2011). Hypertension represents a major risk factor coronary artery disease, and affects approximately 25% of the adult population worldwide(Kearney et al,2005). It is for developing other diseases such as metabolic syndrome,diabetes,renal dysfunction,congestive heart failure, and estimated that by 2025,the incidence of hypertension would increase to 24% in developed countries and to 80% in developing countries(Messerli et al,2007). In recent years,traditional and natural medicines have been extensively used to prevent and heal various cardiovascular problems including hypertension,the pharmacological validation for the use of folk medicinal plants is of great interest for health(Ho and Jie, 2007).
Coreopsis tinctoria Nutt.(CT,Asteraceae)(Figure 1)is a widely distributed medicinal plant in southern region of Xinjiang Uygur Autonomous Region in China(Pan et al,2012), and its dry buds were used in the treatment of hypertension in the Uyghur folk medicine(Cao et al,2011). Previous studies have also revealed that CT had the antihypertensive efficacy on renal hypertensive rats and vasorelaxant effect in vitro(Ling et al,2013b; Sun et al,2013). Although spontaneously hypertensive rats(SHRs)were known as the model for studying hypertension,neither of the previous researches involved the use of CT in SHR,nor the cognizance of the effect of the plant extract on angiotensin II(Ang II),malondialdehyde(MDA),nitric oxide(NO), and expression of various genes in vivo had been found. Therefore,the present study was designed to evaluate the effects of the flavonoids from the flower buds of CT(CT-F)in SHRs and to explore the underlying mechanism of the antihypertensive activity.

| Fig.1Coreopsis tinctoria Nutt. (from http://p0.so.qhimg.com/t0194d310f68e98c4aa.jpg) |
The flower buds of Coreopsis tinctoria Nutt. were collected in June,2012 at Pishan county,Hetian region,Xinjiang Uygur Autonomous Region of China and authenticated by Yang WJ,associate professor of Xinjiang Institute of Materia Medica,China. A voucher specimen(No. 2008-P1CT)has been deposited in the herbarium of Xinjiang Institute of Materia Medica. The radioimmunoassay kits for quantifying Ang II in plasma were provided by Beijing North Institute of Biological Technology(China). Kits for quantifying MDA and NO were purchased from Nanjing Jiancheng Chemical Factory(China). Captopril was obtained from Shantou Jingshi Pharmaceutical Factory(China). Methyl cyanides used for HPLC were of chromatographic grade, and other chemicals used were of analytical grade from commercial sources.
2.2 Extraction and identification of CT-FThe dried and powdered flower buds of C. tinctoria(2.0 kg)were consecutively extracted under reflux twice,with 95% and 50% ethanol respectively, and the solvent was removed by evaporation under reduced pressure using RE–3000 Rotary Evaporator(Yarong Biochemistry Instrument Factory,China)to yield the ethanol extract. The ethanol extract was purified by D-101 resin to obtain 10%,30%,50%,70%, and 95% ethanol extracting eluates,among which 50% ethanol extract(272.0 g,13.6%)were rich in flavonoids. The CT-F(100 mg)was dissolved in 50% ethanol solution. The flavonoids were obtained by ultrasonic extraction for 15 min at room temperature. The sample was filtered on a 0.45 μm nylon membrane filter before injected into LC-20A RP-HPLC(Shimadzu,Japan)on Inertsil ODS-3 C18 column(250 mm × 4.6 mm,5 µm,Shimadzu,Japan)at 25 oC. The running conditions were as follows: injection volume of 10 µL,mobile phase of methyl cyanides-0.4% H3PO4(45:55)on a gradient run,flow rate of 1.0 mL/min, and detection wavelength at 325 nm. The quantitative analysis was performed using external st and ard method. The pure st and ards of luteolin and quercetin(National Institutes for Food and Drug Control)were used as st and ard to identify the compounds, and the other st and ard substances were domestic(purity > 98.0%). The flavonoids presented in the sample were identified by comparing chromatographic peaks with the retention time of individual st and ards and further confirmed by co-injection with isolated st and ards.
2.3 Treatment of SHRs and Wistar-Kyoto ratsTwenty-four SHRs(9–10 weeks old,male,SPF,weighing 240–260 g) and eight age-matched normotensive Wistar-Kyoto rats(WKYRs)(male,SPF,weighing 260–280 g)were purchased from Vital River Experiment Animal,Inc.(China). All the animals were housed in a temperature- controlled [(25 ± 2)oC] room and allowed to acclimate for 1 week prior to the experiments. Rats were housed in groups of two per cage in a room at a relative humidity of 40%–42% with a 12 h light-dark cycle and were fed with a st and ard laboratory diet. Distilled water was freely available to the animals. The systolic blood pressure(SBP)was measured by the tail-cuff method with a tail measurement device(BP-300A,Chengdu TME Technology Co.,Ltd.,China). The protocols were approved by the Ethics Materia Committee on Animal Experiment,Xinjiang Institute of Medica.
The SHRs were divided into model,captopril(40 mg/kg,positive control), and CT-F(100 mg/kg)groups, and the WKYRs were set as control group,eight in each group. The SHRs were ig administered with the appropriate drugs dissolved in distilled water. The SHRs in the model group and WKYRs were ig administered with 10 mL/kg distilled water. The blood pressure was measured respectively before administration and on days 1,2,3,5,7,14,21,28,35, and 42 after administration using the tail-cuff method for all rats.
2.4 Determination of biochemical parametersAfter 6 weeks of drug treatment,the rats were fasted but given free access to water for 12 h before being sacrificed. All the animals were ip anesthetized using pentobarbital sodium(40 mg/kg), and then sacrificed immediately. The blood samples were taken from the abdominal aorta and collected in tubes with anticoagulants and coagulants respectively, and centrifuged for 10 min at 3000 g using 3k30 High Speed Refrigerated Centrifuge(Sigma,USA). The supernatants were collected and then analyzed using radioimmunoassay kits to determine the levels of Ang II by GC-2016γ Radio-immunity Counter(Keda Chuangxin Co.,Ltd.,China)at the Nuclear Medicine Laboratory of Xinjiang Medicine University. The concentration of NO and MDA was determined using commercial kits according to the protocols of manufacturers. The main tissues including thoracic aorta and heart of each rat were immediately excised,adhering tissues being trimmed, and fixed in 10% neutral-buffered formaldehyde for histological study. The specimens were embedded in paraffin, and the sections were stained with hematoxylin-eosin(HE)for light microscopic obsevation. The sections were examined and photographed using an Olympus CX–31 Microscope. Three areas in each slide were r and omly chosen for microscope examination. The slides were further examined and evaluated blindly by two investigators.
2.5 Reverse transcription and polymerase chain reactionThe total RNA was extracted from left ventricles using RNA Pure Tissue Kit(Beijing Cowin Bioscience Co.,Ltd.,China). The reverse transcription reaction was performed using high capacity cDNA synthesis kit(Beijing Cowin,China). The quantitative real time RT-PCR analyses of angiotensin-converting enzyme(ACE),ACEII,angiotensin type 1 receptor(AT1R), and TGF-β1 in rats were performed by using SYBR Green Kit(Fermentas,USA)on a Rotor- Gene Q Real-time Quantitative PCR Analyser(Qiagen,Germany). β-Actin was used for normalization. The thermocycling program was as follows: denaturation at 95 ℃ for 5 min,95 oC for 30 s,60–63 oC for 30 s,72 oC for 30 s for 35 cycles, and then 72 oC for 5 min(Table 1).
| Table 1 Parameters of primer pairs for ACE,ACEII,AT1R,TGF-β1, and β-actin genes |
Histological data were analyzed using IPP 5.0.2 software. Quantitative data were expressed as x±s, and all statistical comparisons were made using a One-way ANOVA test by SPSS 13.0. Differences were considered statistically significant when P < 0.05 or 0.01.
3. Results 3.1 Compounds identification of CT-F by UV and RP-HPLCThe HPLC results of CT-F(Figure 1)showed that the compounds included flavanomarein(1,6.20%),marein(2,3.92%),quercetagetin-7-O-glucoside(3,16.90%),quercetin(4,4.87%),luteolin(5,2.31%), and coreopsis chalcone(6,1.63%),shown in Figure 2.
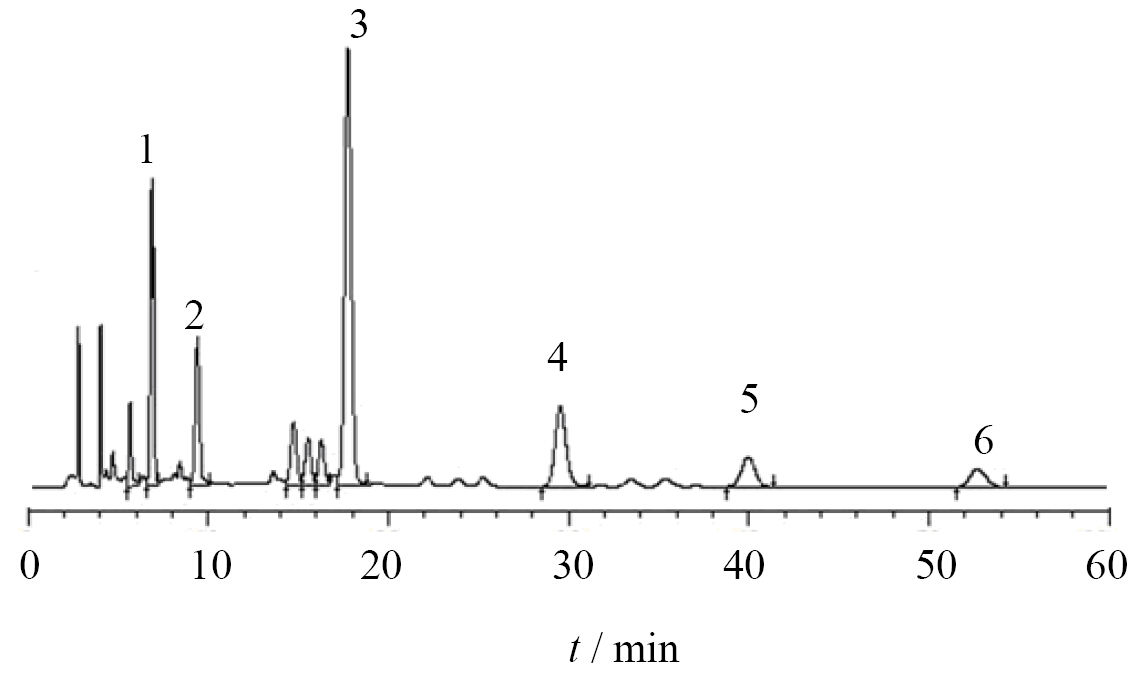
|
Figure 1 HPLC of CT-F 1 : flavanomarein 2: marein 3: quercetagetin-7-O-glucoside 4 : quercetin 5: luteolin 6: coreopsis chalcone |

| Figure 2 Structures of compounds 1–6 |
As shown in Figure 3,the effects of CT-F 100 mg/kg on SBP in SHRs for six weeks were measured. Seen from the mean,systolic blood pressures of SHRs were significantly higher compared with WKYRs before administration; meanwhile there is no difference in SBP among the SHR groups. After ig administration with CT-F(100 mg/kg) and captopril(40 mg/kg)for six consecutive weeks,the SBPs of SHRs were reduced gradually and reached a relatively stable level at last. Furthermore,the SBP of SHRs in the treatment groups were significantly lower than those of SHRs in the model group(P < 0.01).

|
Figure 3 Effect of CT-F on SBP of SHRs (x±s,n=8) *P < 0.05 **P < 0.01 vs model group |
Ang II could raise the blood pressure(BP)through vasoconstriction and salt-water retention. As shown in Table 2,after 6 weeks of treatment,the CT-F group showed lower level of Ang II [(624.96 ± 11.57)pg/mL] in plasma than that of the model group [(1143.42 ± 37.56)pg/mL](P < 0.01).
3.4 Effects of CT-F on NO and MDA in serumAs shown in Table 2,the MDA level in serum of model group was much higher than that of WKYRs(P < 0.05). CT-F improved the MDA activity in serum of SHRs, and the MDA levels of SHRs were significantly decreased compared with the model group(P < 0.05),but captopril decreased the MDA level of SHRs in serum compared with the model group(P > 0.05).
| Table 2 Effects of CT-F on Ang II in plasma and NO and MDA levels in serum of rats(x±s,n=8) |
NO levels were apparently reduced in SHR compared to WKYRs(P < 0.05)(Table 2). However,CT-F treatment restored the NO levels in SHR,which were significantly increased compared with the model group(P < 0.01).
3.5 Effects of CT-F on thoracic aorta histologyThe increase in media thickness is a marker of structural modifications of aorta walls which is directly related to the higher pressure. As shown in Figure 4,thickening of the aortic media was clearly observed in the arteries of SHRs in the model group [(120.13 ± 7.56)μm]. In contrast,the CT-F treated SHR exhibited thinner media thickness [(78.57 ± 4.11)μm](P < 0.05).

| Figure 4 Effects of CT-F on media thickness of aortas in rats(HE staining) |
Hypertension-induced cardiovascular damage could generate destructive oxidants and oxygen free radicals,which could induce myocardium oxidation necroses. As shown in Figure 5,the SHRs were notably ameliorated after treated with CT-F and captopril.

| Figure 5 Effect of CT-F on myocardium oxidation necrosis of SHRs(HE staining) |
As shown in Figure 6,compared with WKYRs group,ACE II in the model group was lower(P < 0.05),but the expression of ACE,AT1R, and TGF-β1 was higher(P < 0.05). Compared with the model group,the expression of ACE II in both captopril and CT-F groups was higher(P < 0.05),but those of ACE,AT1R, and TGF-β1 were lower(P < 0.05).

| Figure 6 Effects of CT-F on mRNA expression of ACE,ACE II,AT1R, and TGF-β1 in left ventricles of SHRs(x±s,n=8) |
The flower buds of Coreopsis tinctoria Nutt.(CT)have demonstrated many beneficial effects,including anti- hypertensive activity and reducing hyperglycemia in diabetic patients(Teresa et al,2010; Luis et al,2011). The ethanol extracts from the flower buds of CT have a high amount of natural flavonoids,including chalcone,flavanone, and flavone(Zhu et al,2006; Jing et al,2012),which are considered to mainly contribute to the health benefits of CT. However,it is important to note that not all flavonoids exhibit the same bioactivity(Shi and Xiao, 2009; Gao et al,2009; Fu et al,1980). This fact necessitates the characterization of the flower buds of CT to identify their bioactive constituents. In this study,the reverse-phase HPLC analysis of the individual CT-F revealed that the most abundant CT-F was quercetagetin-7-O-glucoside(16.90%),successively followed by flavano-marein(6.20%),quercetin(4.87%),marein(3.92%),luteolin(2.31%), and coreopsis chalcones(1.63%).
In this study,SHR was chosen as a model for studying hypertension since the high BP is genetic and idiopathic, and WKYRs were also used specifically as control to SHRs. Based on the previous study,we chose only one dose of CT-F(100 mg/kg),which is the most effective dose in a clinical effective dose range(Ling et al,2013a). Our results showed that daily ig administration ofCT-F caused notable decline in SBP after 3 d and the persistent hypotensive effect was observed in the following 39 d. Meanwhile,CT-F was able to stably lower the plateau SBP. At the end of this experiment,CT-F and captopril groups exert the same therapeutic effect(P > 0.05).
Further observation was conducted to explore the possible mechanisms of CT-F in lowering BP in this study. The association of free radical production,lipid peroxidation,oxidative stress, and hypertension was well known(Martínez-Revelles et al,2013). It was reported that the vascular oxidative stress played a major role in pathogenesis of hypertension(Popolo et al,2013; Sahoko,2013). Hypertension-induced cardio-vascular damage could generate destructive oxidants and oxygen free radicals,which could increase BP through decreasing NO bioavailability and result in reactive oxygen species(ROS)-mediated cardiovascular remodeling(Zhang et al,2010). Previous studies showed that CT exerts anti-oxidative activity that could scavenge the hydroxyl radical(OH),superoxide radical(O2−), and DPPH radical in vitro(Cao et al,2011; Luis et al,2011). The present results showed that treatment with CT-F significantly reduced the MDA level in serum and the thoracic aorta media thickness in SHRs. The MDA level in serum reflected probably general indexes of oxidative status and lipid peroxidation(Dmitriev and Titov, 2010),therefore,the anti- hypertensive effects of CT-F may be associated with a reduced oxidant status due to its anti-oxidative properties.
Previous studies disclosed that the CT had the significant role in dilation on blood vessels(Sun et al,2013). NO played a relaxation role on various blood vessels,which reduced peripheral vascular resistance. Studies showed that NO could diffuse to nearby smooth muscle cells(SMCs) and increase the cytoplasmic level of cyclic guanosine monophosphate(cGMP),which could dilate SMCs via various ways and lead to hypotension(Murad,1986). Our results showed that CT-F administration could significantly elevate the level of NO in serum.
In hypertension,the increased vascular resistance could result in basic haemodynamic abnormality,which was due not only to vasoconstriction,but also to structural changes in the arterial wall(Saleh and Jurjus, 2001). The increase in media thickness is a marker of structural modifications characterized by SMC hypertrophy and fibrosis which is directly related to higher pressure(Simon et al,2002). Pharmacologic treatment in SHRs could attenuate the hypertensive structural changes in large arteries, and this effect is associated with a reduction in BP.(Frantisek et al,2013). The data suggested that CT-F could ameliorate some vascular histopathologic changes,thus the diminished aortic media thickness could be consequences of the reduction in BP.
As a leading cause of death and disability worldwide,the rennin angiotensin system(RAS)plays an important role in the pathophysiology of CVD(Bos,2011). Within the RAS,the ACE converts Ang I into the vasoconstrictor Ang II,the main effector of the system,which mediates its effects via the AT1R. Ang II has the actions to raise BP through vasoconstriction and salt-water retention, and contributes to cardiac remodeling,inflammation,thrombosis, and plaque rupture(Louise et al,2013). The Ang II level was higher in SHRs(Ryohei et al,2013). Recent studies reported that ACE II was an important regulator of cardiac pathophysiology(Yamamoto et al,2006). In this study,the influence of CT-F on the Ang II level and mRNA expression of RAS-related gene in plasma of SHRs were observed. Our experimental data showed that CT-F could down-regulate Ang II in plasma, and the expression of ACE,AT1R, and TGF-β1 in the left ventricles,but up-regulate ACE II.
Hypertension-induced progressive accumulation of interstitial collagen fibers may disrupt myocyte alignment and impair the cardiac contractile function(Lopez et al,2006). In this respect,growing evidence indicates that transforming grown factor-β(TGF-β)is involved in the progression of cardiac hypertrophy to heart failure(Spinale,2007). TGF-β is an important mechanism for the transition of fibroblasts to myofibroblasts,thus promoting fibrosis. The present data show that CT-F has a descent effect on TGF-β mRNA.
| [1] | Bos JL,1989. Ras oncogenes in human cancer−a review. Cancer Res 49: 4682-4689. |
| [2] | Cao Y,Pang SB,Xu L,Fan YD,Ming T,Sun YH,2011. Antioxidant activities of Coreopsis tinctoria extractsin vitro. Chin J Exp Tradit Medl Form 17: 144-147. |
| [3] | Dmitriev LF,Titov VN,2010. Lipid peroxidation in relation to ageing and the role of endogenous aldehydes in diabetes and other age-related diseases. Ageing Res Rev 9: 200-210. |
| [4] | Elen R,Michele MC,Carla SC,Ev and ro MNN,Cibele MP,Marcos AR,Jose ETS,Raquel FG,2013. Tempol inhibits TGF-β and MMPs upregulation and prevents cardiac hypertensive changes. Int J Cardiol 165: 165-173. |
| [5] | Frantisek K,Magdalena M,Sona C,2013. Long-term effect of prazosin and losartan administration on blood pressure,heart,carotid artery, and acetylcholine induced dilation of cardio- vascular system of young Wistar rats and SHR. Gen Physiol Biophys 32: 235-243. |
| [6] | Fu FY,Liu YL,Shang TM,Xiao PG,1980. Distribution of flavonoids,in the plant kingdom,therapeutic values and potential new drugs therefrom. Acta Bot Sin 22: 87-92. |
| [7] | Gao PZ,Li Z,Wu H,Gao ZH,2009. Study on Anti-nitration of six different flavonoids. J Quanzhou Normal Univ(Natural Science) 27: 54-57. |
| [8] | Ho JW,Jie M,2007. Pharmacological activity of cardiovascular agents from herbal medicine. Cardiovasc Hematol Agents Med Chem 5: 273-277. |
| [9] | Kearney PM,Whelton M,Reynolds K,Muntner P,Whelton PK,He J,2005. Global burden of hypertension: Analysis of worldwide data. Lancet 365: 217-223. |
| [10] | Ling B,Hasimu H,Liu XY,Hu MY,Zhang LL,Ma XP,Xu L,Yang Q,Sun YH,2013a. Effects of Coreopsis tinctoria extract on renin-angiotensin-aldoster system in renal hypertensive rats. Chin Pharmacol Bull 29: 1448-1452. |
| [11] | Ling B,Zhang LL,Hasimu H,Liu XY,Hu MY,Ma XP,Xu L,Yang Q,Sun YH,2013b. Effect of Coreopsis tinctoria on blood pressure and renin-angiotensin system in hypertensive mice. Pharmacol Clin Chin Mater Med 29: 80-83. |
| [12] | Lopez B,Gonzalez A,Querejeta R,Larman M,Diez J,2006. Alterations in the pattern of collagen deposition may contribute to the deterioration of systolic function in hypertensive patients with heart failure. J Am Coll Cardiol 48: 89-96. |
| [13] | Louise M,Stephen B,Elena V,Sheila K,2013. The ACE2 gene: It’s potential as a functional c and idate for cardiovascular disease. Clin Sci 124: 65-76. |
| [14] | Luis G,Andreia PO,Luis RS,Paula BA,Paula GP,Joao B,Patricia V,2011. Metabolic and biological prospecting of Coreopsis tinctoria. Rev Bras Farmacogn 22: 350-358. |
| [15] | Martínez-Revelles S,Avendaño MS,Garcia-Redondo AB,Álvarez Y,Aguado A,Pérez-Girón JV,García-Redondo L,Esteban V,Redondo JM,Alonso MJ,Briones AM,Salaices M,2013. Reciprocal relationship between reactive oxygen species and cyclooxygenase-2 and vascular dysfunction in hypertension. Antioxid Redox Signal 18: 51-65. |
| [16] | Messerli FH,Williams B,Ritz E,2007. Essential hypertension. Lancet 370: 591-603. |
| [17] | Murad F,1986. Cyclic guanosine monophosphate as a mediator of vasodilation. J Clin Invest 78: 1-5. |
| [18] | Pan Y,Li N,Ni H,Meng DL,Jia XG,2012. Advantages in research on chemical constituents in plants of Coreopsis L. and their pharmacological activities. Drug Clin 27(5): 512-518. |
| [19] | Popolo A,Autore G,Pinto A,Marzocco S,2013. Oxidative stress in patients with cardiovascular disease and chronic renal failure. Free Radic Res 47: 346-356. |
| [20] | Ryohei K,Yoshiharu O,Misa N,Ken-ichi I,Akihiko T,Miki Y,Ryu H,Hirotoshi U,2013. Peach(Prunus persica)extract inhibits angiotensin II-induced signal transduction in vascular smooth muscle cells. Food Chem 139: 371-376. |
| [21] | Sahoko I,2013. The pathological roles of environmental and redox stresses in cardiovascular disease. Environ Health Prev Med 18: 177-184. |
| [22] | Saleh FH,Jurjus AR,2001. A comparative study of morphological changes in spontaneously hypertensive rats and normotensive Wistar Kyoto rats treated with an angiotensin-converting enzyme inhibitor or a calcium-channel blocker. J Pathol 193: 415-420. |
| [23] | Shi JY,Xiao Y,2009. Studies on anti-oxidative activity of different polarity flavonoids from Caulis Polygoni Multiflori extract. Food Ferment Technol 45: 35-37. |
| [24] | Simon A,Gariepy J,Chironi G,Megnien JL,Levenson J,2002. Intima-media thickness: a new tool for diagnosis and treatment of cardiovascular risk. J Hypertension 20: 159-169. |
| [25] | Spinale FG,2007. Myocardial matrix remodeling and the matrix metalloproteinases: Influence on cardiac form and function. Physiol Rev 87: 1285-1342. |
| [26] | Sun YH,Zhao J,Jin HT,Cao Y,Ming T,Zhang LL,Hu MY,Hasimu H,Pang SB,Ma XP,2013. Vasorelaxant effects of the extracts and some flavonoids from the buds of Coreopsis tinctoria. Pharm Biol 51(9): 1158-1164. |
| [27] | Tang YQ,Wang MH,Chen CL,Le XY,Sun SJ,Yin YM,2011. Cardiovascular protection with danshensu in spontaneously hypertensive rats. Biol Pharm Bull 34: 1596-1601. |
| [28] | Teresa D,Maria RB,Peter JH,Helder MF,Alex and ra P,2010. The flavonoid-rich fraction of Coreopsis tinctoria promotes glucose tolerance regain through pancreatic function recovery in streptozotocin-induced glucose-intolerant rats. J Ethnopharmacol 132: 483-490. |
| [29] | Yamamoto K,Ohishi M,Katsuya T,Ito N,Ikushima M,Kaibe M,Tatara Y,Shiota A,Sugano S,Takeda S,Rakugi H,Ogihara T,2006. Deletion of angiotensin-converting enzyme 2 accelerates pressure overload-induced cardiac dysfunction by increasing local angiotensin II. Hypertension 47(4): 718-726. |
| [30] | Yang C,Liu XX,Li SN,2010. Effect of long-term treatment with urocortin on the activity of somatic angiotensin-converting enzyme in spontaneously hypertensive rats. Can J Physiol Pharmacol 88: 168-176. |
| [31] | Zhang G.S,Wang RJ,Zhang HN,Zhang GP,Luo MS,Luo JD,2010. Effects of chronic treatment with honokiol in spontaneously hypertensive rats. Biol Pharm Bull 33: 427-431. |
| [32] | Zhao J,Sun YH,Xu F,Li CY,Hu MY,Zhang LL,Pang SB,2013. Study on flavonoids of Coreopsis tinctoria Nutt. Nat Prod Res Dev 25: 50-52. |
 2014,Vol. 6
2014,Vol. 6


